[English] 日本語
 Yorodumi
Yorodumi- EMDB-32011: Asymmetric unit of cryoEM structure of bacteriophage lambda capsi... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-32011 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Asymmetric unit of cryoEM structure of bacteriophage lambda capsid at 3.76 Angstrom | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | bacteriophage lambda / capsid / procapsid / capsid maturation / virus structure / cryo-EM / auxiliary protein / conformational expansion / cementing protein / DNA packaging / VIRUS | |||||||||
| Function / homology |  Function and homology information Function and homology informationviral capsid, decoration / viral DNA genome packaging / T=7 icosahedral viral capsid / viral capsid / host cell cytoplasm Similarity search - Function | |||||||||
| Biological species |  Escherichia phage lambda (virus) / Escherichia phage lambda (virus) /  Escherichia virus Lambda Escherichia virus Lambda | |||||||||
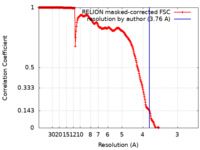
| Method | single particle reconstruction / cryo EM / Resolution: 3.76 Å | |||||||||
 Authors Authors | Wang JW | |||||||||
| Funding support |  China, 2 items China, 2 items
| |||||||||
 Citation Citation |  Journal: Structure / Year: 2022 Journal: Structure / Year: 2022Title: Structural basis of bacteriophage lambda capsid maturation. Authors: Chang Wang / Jianwei Zeng / Jiawei Wang /  Abstract: Bacteriophage lambda is an excellent model system for studying capsid assembly of double-stranded DNA (dsDNA) bacteriophages, some dsDNA archaeal viruses, and herpesviruses. HK97 fold coat proteins ...Bacteriophage lambda is an excellent model system for studying capsid assembly of double-stranded DNA (dsDNA) bacteriophages, some dsDNA archaeal viruses, and herpesviruses. HK97 fold coat proteins initially assemble into a precursor capsid (procapsid) and subsequent genome packaging triggers morphological expansion of the shell. An auxiliary protein is required to stabilize the expanded capsid structure. To investigate the capsid maturation mechanism, we determined the cryo-electron microscopy structures of the bacteriophage lambda procapsid and mature capsid at 3.88 Å and 3.76 Å resolution, respectively. Besides primarily rigid body movements of common features of the major capsid protein gpE, large-scale structural rearrangements of other domains occur simultaneously. Assembly of intercapsomers within the procapsid is facilitated by layer-stacking effects at 3-fold vertices. Upon conformational expansion of the capsid shell, the missing top layer is fulfilled by cementing the gpD protein against the internal pressure of DNA packaging. Our structures illuminate the assembly mechanisms of dsDNA viruses. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_32011.map.gz emd_32011.map.gz | 64.8 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-32011-v30.xml emd-32011-v30.xml emd-32011.xml emd-32011.xml | 15.3 KB 15.3 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_32011_fsc.xml emd_32011_fsc.xml | 25.4 KB | Display |  FSC data file FSC data file |
| Images |  emd_32011.png emd_32011.png | 203.6 KB | ||
| Filedesc metadata |  emd-32011.cif.gz emd-32011.cif.gz | 5.8 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-32011 http://ftp.pdbj.org/pub/emdb/structures/EMD-32011 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-32011 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-32011 | HTTPS FTP |
-Related structure data
| Related structure data |  7vikMC  7vi9C  7viaC  7viiC C: citing same article ( M: atomic model generated by this map |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_32011.map.gz / Format: CCP4 / Size: 1.4 GB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_32011.map.gz / Format: CCP4 / Size: 1.4 GB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.30654 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
- Sample components
Sample components
-Entire : Escherichia virus Lambda
| Entire | Name:  Escherichia virus Lambda Escherichia virus Lambda |
|---|---|
| Components |
|
-Supramolecule #1: Escherichia virus Lambda
| Supramolecule | Name: Escherichia virus Lambda / type: virus / ID: 1 / Parent: 0 / Macromolecule list: all / NCBI-ID: 10710 / Sci species name: Escherichia virus Lambda / Virus type: VIRUS-LIKE PARTICLE / Virus isolate: OTHER / Virus enveloped: No / Virus empty: Yes |
|---|
-Macromolecule #1: Major capsid protein
| Macromolecule | Name: Major capsid protein / type: protein_or_peptide / ID: 1 / Number of copies: 7 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Escherichia phage lambda (virus) Escherichia phage lambda (virus) |
| Molecular weight | Theoretical: 38.22916 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MSMYTTAQLL AANEQKFKFD PLFLRLFFRE SYPFTTEKVY LSQIPGLVNM ALYVSPIVSG EVIRSRGGST SEFTPGYVKP KHEVNPQMT LRRLPDEDPQ NLADPAYRRR RIIMQNMRDE ELAIAQVEEM QAVSAVLKGK YTMTGEAFDP VEVDMGRSEE N NITQSGGT ...String: MSMYTTAQLL AANEQKFKFD PLFLRLFFRE SYPFTTEKVY LSQIPGLVNM ALYVSPIVSG EVIRSRGGST SEFTPGYVKP KHEVNPQMT LRRLPDEDPQ NLADPAYRRR RIIMQNMRDE ELAIAQVEEM QAVSAVLKGK YTMTGEAFDP VEVDMGRSEE N NITQSGGT EWSKRDKSTY DPTDDIEAYA LNASGVVNII VFDPKGWALF RSFKAVKEKL DTRRGSNSEL ETAVKDLGKA VS YKGMYGD VAIVVYSGQY VENGVKKNFL PDNTMVLGNT QARGLRTYGC IQDADAQREG INASARYPKN WVTTGDPARE FTM IQSAPL MLLADPDEFV SVQLA UniProtKB: Major capsid protein |
-Macromolecule #2: Capsid decoration protein
| Macromolecule | Name: Capsid decoration protein / type: protein_or_peptide / ID: 2 / Number of copies: 7 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Escherichia phage lambda (virus) Escherichia phage lambda (virus) |
| Molecular weight | Theoretical: 11.582873 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MTSKETFTHY QPQGNSDPAH TATAPGGLSA KAPAMTPLML DTSSRKLVAW DGTTDGAAVG ILAVAADQTS TTLTFYKSGT FRYEDVLWP EAASDETKKR TAFAGTAISI V UniProtKB: Capsid decoration protein |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.4 |
|---|---|
| Vitrification | Cryogen name: NITROGEN |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Average electron dose: 50.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller












 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)