+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-3179 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo-EM structure of yeast apo RNA polymerase III at 4.6 A | |||||||||
 Map data Map data | Reconstruction of apo RNA polymerase III in the closed clamp conformation | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | RNA polymerase III / pol III / transcription / RNA polymerase | |||||||||
| Function / homology |  Function and homology information Function and homology informationRNA Polymerase I Transcription Initiation / Processing of Capped Intron-Containing Pre-mRNA / RNA Polymerase III Transcription Initiation From Type 2 Promoter / RNA Pol II CTD phosphorylation and interaction with CE / Formation of the Early Elongation Complex / mRNA Capping / RNA polymerase II transcribes snRNA genes / TP53 Regulates Transcription of DNA Repair Genes / RNA Polymerase II Promoter Escape / RNA Polymerase II Transcription Pre-Initiation And Promoter Opening ...RNA Polymerase I Transcription Initiation / Processing of Capped Intron-Containing Pre-mRNA / RNA Polymerase III Transcription Initiation From Type 2 Promoter / RNA Pol II CTD phosphorylation and interaction with CE / Formation of the Early Elongation Complex / mRNA Capping / RNA polymerase II transcribes snRNA genes / TP53 Regulates Transcription of DNA Repair Genes / RNA Polymerase II Promoter Escape / RNA Polymerase II Transcription Pre-Initiation And Promoter Opening / RNA Polymerase II Transcription Initiation / RNA Polymerase II Transcription Initiation And Promoter Clearance / termination of RNA polymerase III transcription / RNA Polymerase II Pre-transcription Events / RNA-templated transcription / Formation of TC-NER Pre-Incision Complex / RNA Polymerase I Promoter Escape / transcription initiation at RNA polymerase III promoter / termination of RNA polymerase I transcription / Gap-filling DNA repair synthesis and ligation in TC-NER / nucleolar large rRNA transcription by RNA polymerase I / transcription initiation at RNA polymerase I promoter / Estrogen-dependent gene expression / transcription by RNA polymerase III / Dual incision in TC-NER / RNA polymerase I complex / transcription elongation by RNA polymerase I / RNA polymerase III complex / tRNA transcription by RNA polymerase III / RNA polymerase II, core complex / transcription by RNA polymerase I / nucleotidyltransferase activity / transcription initiation at RNA polymerase II promoter / transcription elongation by RNA polymerase II / ribonucleoside binding / DNA-directed RNA polymerase / DNA-directed RNA polymerase activity / peroxisome / single-stranded DNA binding / ribosome biogenesis / nucleic acid binding / transcription by RNA polymerase II / protein dimerization activity / nucleotide binding / nucleolus / mitochondrion / DNA binding / zinc ion binding / nucleoplasm / metal ion binding / nucleus / cytoplasm / cytosol Similarity search - Function | |||||||||
| Biological species |  | |||||||||
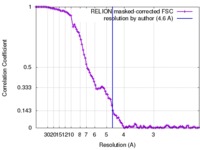
| Method | single particle reconstruction / cryo EM / Resolution: 4.6 Å | |||||||||
 Authors Authors | Hoffmann NA / Jakobi AJ / Moreno-Morcillo M / Glatt S / Kosinski J / Hagen WJ / Sachse C / Muller CW | |||||||||
 Citation Citation |  Journal: Nature / Year: 2015 Journal: Nature / Year: 2015Title: Molecular structures of unbound and transcribing RNA polymerase III. Authors: Niklas A Hoffmann / Arjen J Jakobi / María Moreno-Morcillo / Sebastian Glatt / Jan Kosinski / Wim J H Hagen / Carsten Sachse / Christoph W Müller /  Abstract: Transcription of genes encoding small structured RNAs such as transfer RNAs, spliceosomal U6 small nuclear RNA and ribosomal 5S RNA is carried out by RNA polymerase III (Pol III), the largest yet ...Transcription of genes encoding small structured RNAs such as transfer RNAs, spliceosomal U6 small nuclear RNA and ribosomal 5S RNA is carried out by RNA polymerase III (Pol III), the largest yet structurally least characterized eukaryotic RNA polymerase. Here we present the cryo-electron microscopy structures of the Saccharomyces cerevisiae Pol III elongating complex at 3.9 Å resolution and the apo Pol III enzyme in two different conformations at 4.6 and 4.7 Å resolution, respectively, which allow the building of a 17-subunit atomic model of Pol III. The reconstructions reveal the precise orientation of the C82-C34-C31 heterotrimer in close proximity to the stalk. The C53-C37 heterodimer positions residues involved in transcription termination close to the non-template DNA strand. In the apo Pol III structures, the stalk adopts different orientations coupled with closed and open conformations of the clamp. Our results provide novel insights into Pol III-specific transcription and the adaptation of Pol III towards its small transcriptional targets. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_3179.map.gz emd_3179.map.gz | 11.5 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-3179-v30.xml emd-3179-v30.xml emd-3179.xml emd-3179.xml | 9 KB 9 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_3179_fsc.xml emd_3179_fsc.xml | 10.3 KB | Display |  FSC data file FSC data file |
| Images |  emd_3179.jpg emd_3179.jpg | 66.4 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-3179 http://ftp.pdbj.org/pub/emdb/structures/EMD-3179 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-3179 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-3179 | HTTPS FTP |
-Related structure data
| Related structure data |  5fj9MC  3178C  3180C  5fj8C  5fjaC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_3179.map.gz / Format: CCP4 / Size: 96.6 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_3179.map.gz / Format: CCP4 / Size: 96.6 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Reconstruction of apo RNA polymerase III in the closed clamp conformation | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.084 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
- Sample components
Sample components
-Entire : S.cerevisiae apo RNA polymerase III (closed clamp conformation)
| Entire | Name: S.cerevisiae apo RNA polymerase III (closed clamp conformation) |
|---|---|
| Components |
|
-Supramolecule #1000: S.cerevisiae apo RNA polymerase III (closed clamp conformation)
| Supramolecule | Name: S.cerevisiae apo RNA polymerase III (closed clamp conformation) type: sample / ID: 1000 / Oligomeric state: Monomer / Number unique components: 17 |
|---|---|
| Molecular weight | Theoretical: 690 KDa |
-Macromolecule #1: RNA polymerase III
| Macromolecule | Name: RNA polymerase III / type: protein_or_peptide / ID: 1 / Number of copies: 1 / Oligomeric state: Monomer / Recombinant expression: No |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Experimental: 690 KDa |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 0.2 mg/mL |
|---|---|
| Buffer | pH: 7.5 / Details: 150mM (NH4)2SO4, 15mM Tris, 10mM DTT |
| Grid | Details: Mo 400 mesh Quantifoil 1.2/1.3 |
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 95 % / Instrument: FEI VITROBOT MARK III Method: 2.5 ul of sample was applied 15 seconds wait time Blot for 9 seconds before plunging |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Date | Dec 15, 2014 |
| Image recording | Category: CCD / Film or detector model: FEI FALCON II (4k x 4k) / Number real images: 2398 / Average electron dose: 42 e/Å2 Details: Seven frames were recorded with a dose of 6 e/A2 per frame. |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm / Nominal defocus max: 4.2 µm / Nominal defocus min: 1.4 µm / Nominal magnification: 75000 |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller




















 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)