[English] 日本語
 Yorodumi
Yorodumi- EMDB-31556: Complex of FMDV A/AF/72 and bovine neutralizing scFv antibody R55 -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-31556 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Complex of FMDV A/AF/72 and bovine neutralizing scFv antibody R55 | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | FOOT AND MOUTH DISEASE VIRUS / FMDV / VIRUS | |||||||||
| Function / homology |  Function and homology information Function and homology informationsymbiont-mediated perturbation of host chromatin organization / ribonucleoside triphosphate phosphatase activity / T=pseudo3 icosahedral viral capsid / host cell cytoplasmic vesicle membrane / channel activity / monoatomic ion transmembrane transport / clathrin-dependent endocytosis of virus by host cell / RNA helicase activity / viral protein processing / host cell endoplasmic reticulum membrane ...symbiont-mediated perturbation of host chromatin organization / ribonucleoside triphosphate phosphatase activity / T=pseudo3 icosahedral viral capsid / host cell cytoplasmic vesicle membrane / channel activity / monoatomic ion transmembrane transport / clathrin-dependent endocytosis of virus by host cell / RNA helicase activity / viral protein processing / host cell endoplasmic reticulum membrane / cysteine-type endopeptidase activity / viral RNA genome replication / RNA-directed RNA polymerase activity / DNA-templated transcription / virion attachment to host cell / host cell nucleus / structural molecule activity / proteolysis / RNA binding / ATP binding / membrane Similarity search - Function | |||||||||
| Biological species |   Foot-and-mouth disease virus / Foot-and-mouth disease virus /  | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 3.91 Å | |||||||||
 Authors Authors | Yong H / Kun L | |||||||||
 Citation Citation |  Journal: J Virol / Year: 2021 Journal: J Virol / Year: 2021Title: Structures of Foot-and-Mouth Disease Virus with Bovine Neutralizing Antibodies Reveal the Determinant of Intraserotype Cross-Neutralization. Authors: Yong He / Kun Li / Li Wang / Zixian Sun / Yimei Cao / Pinghua Li / Pu Sun / Huifang Bao / Shasha Zhou / Sheng Wang / Xingwen Bai / Xuerong Liu / Lixia Zhao / Xiuli Fan / Zaixin Liu / Zengjun ...Authors: Yong He / Kun Li / Li Wang / Zixian Sun / Yimei Cao / Pinghua Li / Pu Sun / Huifang Bao / Shasha Zhou / Sheng Wang / Xingwen Bai / Xuerong Liu / Lixia Zhao / Xiuli Fan / Zaixin Liu / Zengjun Lu / Cheng Yang / Zhiyong Lou /  Abstract: Foot-and-mouth disease virus (FMDV) exhibits broad antigenic diversity with poor intraserotype cross-neutralizing activity. Studies of the determinant involved in this diversity are essential for the ...Foot-and-mouth disease virus (FMDV) exhibits broad antigenic diversity with poor intraserotype cross-neutralizing activity. Studies of the determinant involved in this diversity are essential for the development of broadly protective vaccines. In this work, we isolated a bovine antibody, designated R55, that displays cross-reaction with both FMDV A/AF/72 (hereafter named FMDV-AAF) and FMDV A/WH/09 (hereafter named FMDV-AWH) but only has a neutralizing effect on FMDV-AWH. Near-atomic resolution structures of FMDV-AAF-R55 and FMDV-AWH-R55 show that R55 engages the capsids of both FMDV-AAF and FMDV-AWH near the icosahedral 3-fold axis and binds to the βB and BC/HI-loops of VP2 and to the B-B knob of VP3. The common interaction residues are highly conserved, which is the major determinant for cross-reaction with both FMDV-AAF and FMDV-AWH. In addition, the cryo-EM structure of the FMDV-AWH-R55 complex also shows that R55 binds to E70 located at the VP3 BC-loop in an adjacent pentamer, which enhances the acid and thermal stabilities of the viral capsid. This may prevent capsid dissociation and genome release into host cells, eventually leading to neutralization of the viral infection. In contrast, R55 binds only to the FMDV-AAF capsid within one pentamer due to the E70G variation, which neither enhances capsid stability nor neutralizes FMDV-AAF infection. The E70G mutation is the major determinant involved in the neutralizing differences between FMDV-AWH and FMDV-AAF. The crucial amino acid E70 is a key component of the neutralizing epitopes, which may aid in the development of broadly protective vaccines. Foot-and-mouth disease virus (FMDV) causes a highly contagious and economically devastating disease in cloven-hoofed animals, and neutralizing antibodies play critical roles in the defense against viral infections. Here, we isolated a bovine antibody (R55) using the single B cell antibody isolation technique. Enzyme-linked immunosorbent assays (ELISA) and virus neutralization tests (VNT) showed that R55 displays cross-reactions with both FMDV-AWH and FMDV-AAF but only has a neutralizing effect on FMDV-AWH. Cryo-EM structures, fluorescence-based thermal stability assays and acid stability assays showed that R55 engages the capsid of FMDV-AWH near the icosahedral 3-fold axis and informs an interpentamer epitope, which overstabilizes virions to hinder capsid dissociation to release the genome, eventually leading to neutralization of viral infection. The crucial amino acid E70 forms a key component of neutralizing epitopes, and the determination of the E70G mutation involved in the neutralizing differences between FMDV-AWH and FMDV-AAF could aid in the development of broadly protective vaccines. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_31556.map.gz emd_31556.map.gz | 97.1 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-31556-v30.xml emd-31556-v30.xml emd-31556.xml emd-31556.xml | 15.5 KB 15.5 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_31556.png emd_31556.png | 247 KB | ||
| Filedesc metadata |  emd-31556.cif.gz emd-31556.cif.gz | 5.8 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-31556 http://ftp.pdbj.org/pub/emdb/structures/EMD-31556 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-31556 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-31556 | HTTPS FTP |
-Related structure data
| Related structure data |  7fejMC  7feiC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_31556.map.gz / Format: CCP4 / Size: 421.9 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_31556.map.gz / Format: CCP4 / Size: 421.9 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.93 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
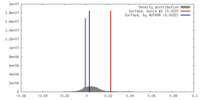
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
- Sample components
Sample components
-Entire : Complex of FMDV A/AF/72 and bovine neutralizing scFv antibody R55
| Entire | Name: Complex of FMDV A/AF/72 and bovine neutralizing scFv antibody R55 |
|---|---|
| Components |
|
-Supramolecule #1: Complex of FMDV A/AF/72 and bovine neutralizing scFv antibody R55
| Supramolecule | Name: Complex of FMDV A/AF/72 and bovine neutralizing scFv antibody R55 type: complex / ID: 1 / Parent: 0 / Macromolecule list: all |
|---|---|
| Source (natural) | Organism:   Foot-and-mouth disease virus Foot-and-mouth disease virus |
-Supramolecule #2: FMDV A/AF/72
| Supramolecule | Name: FMDV A/AF/72 / type: complex / ID: 2 / Parent: 1 / Macromolecule list: #1-#4 |
|---|---|
| Source (natural) | Organism:  |
-Supramolecule #3: and bovine neutralizing scFv antibody R55
| Supramolecule | Name: and bovine neutralizing scFv antibody R55 / type: complex / ID: 3 / Parent: 1 / Macromolecule list: #5-#6 |
|---|
-Macromolecule #1: A/AF/72 VP1
| Macromolecule | Name: A/AF/72 VP1 / type: protein_or_peptide / ID: 1 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:   Foot-and-mouth disease virus Foot-and-mouth disease virus |
| Molecular weight | Theoretical: 23.417545 KDa |
| Sequence | String: TTTTGESADP VTTTVENYGG ETQVQRRQHT NVGFIMDRFV KIPSQSPTHV IDLMQTHQHG LVGALLRAAT YYFSDLEIVV RHDDNLTWV PNGAPETALH NTSNPTAYRK GPFTRLALPY TAPHRVLATV YNGTTKYSTG NAGRRGDLGS LAARVAAQLP A SFNFGAIR ...String: TTTTGESADP VTTTVENYGG ETQVQRRQHT NVGFIMDRFV KIPSQSPTHV IDLMQTHQHG LVGALLRAAT YYFSDLEIVV RHDDNLTWV PNGAPETALH NTSNPTAYRK GPFTRLALPY TAPHRVLATV YNGTTKYSTG NAGRRGDLGS LAARVAAQLP A SFNFGAIR ATVIHELLVR MKRAELYCPR PLLAVEVTSQ DRHKQRIIAP AKQL |
-Macromolecule #2: A/AF/72 VP2
| Macromolecule | Name: A/AF/72 VP2 / type: protein_or_peptide / ID: 2 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:   Foot-and-mouth disease virus Foot-and-mouth disease virus |
| Molecular weight | Theoretical: 24.561641 KDa |
| Sequence | String: DKKTEETTLL EDRILTTRNG HTTSTTQSSV GVTYGYSTGE DHVSGPNTSG LETRVTQAER FFKKHLFDWT TDKAFGHLEK LELPTDHKG VYGHLVDSFA YMRNGWDVEV SAVGNQFNGG CLLVAMVPEY KDFTLREKYQ LTLFPHQFIS PRTNMTAHIT V PYLGVNRY ...String: DKKTEETTLL EDRILTTRNG HTTSTTQSSV GVTYGYSTGE DHVSGPNTSG LETRVTQAER FFKKHLFDWT TDKAFGHLEK LELPTDHKG VYGHLVDSFA YMRNGWDVEV SAVGNQFNGG CLLVAMVPEY KDFTLREKYQ LTLFPHQFIS PRTNMTAHIT V PYLGVNRY DQYKKHKPWT LVVMVLSPLT VNNSGAGQIK VYANIAPTYV HVAGELPSKE |
-Macromolecule #3: A/AF/72 VP3
| Macromolecule | Name: A/AF/72 VP3 / type: protein_or_peptide / ID: 3 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:   Foot-and-mouth disease virus Foot-and-mouth disease virus |
| Molecular weight | Theoretical: 24.142053 KDa |
| Sequence | String: GIVPVACSAG YGGLVTTDPK TADPIYGMVY NPPRTNYPGR FTNLLDVAEA CPTFLCFDDG KPYVVTRADG QRLLAKFDVS LAAKHMSNT YLSGIAQYYA QYSGTINLHF MFTGPTDSKA RYMVAYVPPG METPPDTPEE AAHCIHAEWD TGLNSKFTFS I PYVSAADY ...String: GIVPVACSAG YGGLVTTDPK TADPIYGMVY NPPRTNYPGR FTNLLDVAEA CPTFLCFDDG KPYVVTRADG QRLLAKFDVS LAAKHMSNT YLSGIAQYYA QYSGTINLHF MFTGPTDSKA RYMVAYVPPG METPPDTPEE AAHCIHAEWD TGLNSKFTFS I PYVSAADY AYTASDVAET TNVQGWVCIY QITHGKAEDD TLVVSLSAGK DFELRLPIDP RSQ |
-Macromolecule #4: Capsid protein VP0
| Macromolecule | Name: Capsid protein VP0 / type: protein_or_peptide / ID: 4 / Number of copies: 1 / Enantiomer: LEVO / EC number: RNA-directed RNA polymerase |
|---|---|
| Source (natural) | Organism: Foot-and-mouth disease virus |
| Molecular weight | Theoretical: 8.805154 KDa |
| Sequence | String: GAGQSSPATG SQNQSGNTGS IINNYYMQQY QNSMDTQLGD NAISGGSNEG STDTTSTHTN NTQNNDWFSK LASSAFTGLF GALLA UniProtKB: Genome polyprotein |
-Macromolecule #5: IG HEAVY CHAIN VARIABLE REGION
| Macromolecule | Name: IG HEAVY CHAIN VARIABLE REGION / type: protein_or_peptide / ID: 5 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 13.615155 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: QVQLRESGPS LVKPSQTLSL TCTVSGFSLS DYAVGWVRQA PGKALEFLGS ISTGGNTGYN PALKSRLSIT KDNSKNQVSL SLSSVTTED TATYYCTKSI HSYSVFEYTY MQYVDAWGQG LLVPVSS |
-Macromolecule #6: IG LAMDA CHAIN VARIABLE REGION
| Macromolecule | Name: IG LAMDA CHAIN VARIABLE REGION / type: protein_or_peptide / ID: 6 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 12.88685 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: WAQAVLTQPS SVSGSLGQRV SITCSGSSNN IGRYDVGWYQ QIPGSGLRTI IYASKNRPSG VPDRFSGSRS GNTATLTISS LQAEDEADY FCATGDYSSS TSVFGSGTTL TVLGDYKDDD DKGG |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.4 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TECNAI ARCTICA |
|---|---|
| Image recording | Film or detector model: DIRECT ELECTRON DE-16 (4k x 4k) / Average electron dose: 25.0 e/Å2 |
| Electron beam | Acceleration voltage: 200 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD |
| Experimental equipment |  Model: Talos Arctica / Image courtesy: FEI Company |
- Image processing
Image processing
| Startup model | Type of model: EMDB MAP |
|---|---|
| Final reconstruction | Resolution.type: BY AUTHOR / Resolution: 3.91 Å / Resolution method: FSC 0.143 CUT-OFF / Number images used: 25317 |
| Initial angle assignment | Type: ANGULAR RECONSTITUTION |
| Final angle assignment | Type: ANGULAR RECONSTITUTION |
 Movie
Movie Controller
Controller















 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)