+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-31482 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo-EM structure of the human TACAN channel in a closed state | |||||||||
 Map data Map data | EM map | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | dimer / MEMBRANE PROTEIN | |||||||||
| Function / homology |  Function and homology information Function and homology informationcoenzyme A binding / protein heterooligomerization / nuclear inner membrane / fat cell differentiation / monoatomic ion channel activity / detection of mechanical stimulus involved in sensory perception of pain / antiviral innate immune response / protein homooligomerization / monoatomic ion transmembrane transport / endoplasmic reticulum ...coenzyme A binding / protein heterooligomerization / nuclear inner membrane / fat cell differentiation / monoatomic ion channel activity / detection of mechanical stimulus involved in sensory perception of pain / antiviral innate immune response / protein homooligomerization / monoatomic ion transmembrane transport / endoplasmic reticulum / membrane / plasma membrane Similarity search - Function | |||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 3.66 Å | |||||||||
 Authors Authors | Chen XZ / Wang YJ | |||||||||
| Funding support |  China, 1 items China, 1 items
| |||||||||
 Citation Citation |  Journal: Cell Rep / Year: 2022 Journal: Cell Rep / Year: 2022Title: Cryo-EM structure of the human TACAN in a closed state. Authors: Xiaozhe Chen / Yaojie Wang / Yang Li / Xuhang Lu / Jianan Chen / Ming Li / Tianlei Wen / Ning Liu / Shenghai Chang / Xing Zhang / Xue Yang / Yuequan Shen /  Abstract: TACAN is an ion channel-like protein that may be involved in sensing mechanical pain. Here, we present the cryo-electron microscopic structure of human TACAN (hTACAN). hTACAN forms a dimer in which ...TACAN is an ion channel-like protein that may be involved in sensing mechanical pain. Here, we present the cryo-electron microscopic structure of human TACAN (hTACAN). hTACAN forms a dimer in which each protomer consists of a transmembrane globular domain (TMD) containing six helices and an intracellular domain (ICD) containing two helices. Molecular dynamic simulations suggest that each protomer contains a putative ion conduction pore. A single-point mutation of the key residue Met207 greatly increases membrane pressure-activated currents. In addition, each hTACAN subunit binds one cholesterol molecule. Our data show the molecular assembly of hTACAN and suggest that wild-type hTACAN is in a closed state. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_31482.map.gz emd_31482.map.gz | 43.7 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-31482-v30.xml emd-31482-v30.xml emd-31482.xml emd-31482.xml | 11.7 KB 11.7 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_31482.png emd_31482.png | 119.3 KB | ||
| Filedesc metadata |  emd-31482.cif.gz emd-31482.cif.gz | 5.2 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-31482 http://ftp.pdbj.org/pub/emdb/structures/EMD-31482 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-31482 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-31482 | HTTPS FTP |
-Validation report
| Summary document |  emd_31482_validation.pdf.gz emd_31482_validation.pdf.gz | 475.7 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_31482_full_validation.pdf.gz emd_31482_full_validation.pdf.gz | 475.3 KB | Display | |
| Data in XML |  emd_31482_validation.xml.gz emd_31482_validation.xml.gz | 6 KB | Display | |
| Data in CIF |  emd_31482_validation.cif.gz emd_31482_validation.cif.gz | 6.9 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-31482 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-31482 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-31482 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-31482 | HTTPS FTP |
-Related structure data
| Related structure data |  7f6vMC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_31482.map.gz / Format: CCP4 / Size: 46.4 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_31482.map.gz / Format: CCP4 / Size: 46.4 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | EM map | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.014 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
- Sample components
Sample components
-Entire : Cryo-EM structure of the human TACAN channel in a closed state
| Entire | Name: Cryo-EM structure of the human TACAN channel in a closed state |
|---|---|
| Components |
|
-Supramolecule #1: Cryo-EM structure of the human TACAN channel in a closed state
| Supramolecule | Name: Cryo-EM structure of the human TACAN channel in a closed state type: organelle_or_cellular_component / ID: 1 / Parent: 0 / Macromolecule list: #1 |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
-Macromolecule #1: Ion channel TACAN
| Macromolecule | Name: Ion channel TACAN / type: protein_or_peptide / ID: 1 / Number of copies: 2 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 40.657156 KDa |
| Recombinant expression | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: MQPPPPGPLG DCLRDWEDLQ QDFQNIQETH RLYRLKLEEL TKLQNNCTSS ITRQKKRLQE LALALKKCKP SLPAEAEGAA QELENQMKE RQGLFFDMEA YLPKKNGLYL SLVLGNVNVT LLSKQAKFAY KDEYEKFKLY LTIILILISF TCRFLLNSRV T DAAFNFLL ...String: MQPPPPGPLG DCLRDWEDLQ QDFQNIQETH RLYRLKLEEL TKLQNNCTSS ITRQKKRLQE LALALKKCKP SLPAEAEGAA QELENQMKE RQGLFFDMEA YLPKKNGLYL SLVLGNVNVT LLSKQAKFAY KDEYEKFKLY LTIILILISF TCRFLLNSRV T DAAFNFLL VWYYCTLTIR ESILINNGSR IKGWWVFHHY VSTFLSGVML TWPDGLMYQK FRNQFLSFSM YQSFVQFLQY YY QSGCLYR LRALGERHTM DLTVEGFQSW MWRGLTFLLP FLFFGHFWQL FNALTLFNLA QDPQCKEWQV LMCGFPFLLL FLG NFFTTL RVVHHKFHSQ RHGSKKD UniProtKB: Transmembrane protein 120A |
-Macromolecule #2: CHOLESTEROL
| Macromolecule | Name: CHOLESTEROL / type: ligand / ID: 2 / Number of copies: 2 / Formula: CLR |
|---|---|
| Molecular weight | Theoretical: 386.654 Da |
| Chemical component information |  ChemComp-CLR: |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.5 |
|---|---|
| Grid | Model: C-flat-2/1 / Material: GOLD / Pretreatment - Type: GLOW DISCHARGE |
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Instrument: FEI VITROBOT MARK IV |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Detector mode: SUPER-RESOLUTION / Average electron dose: 56.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: 2.0 µm / Nominal defocus min: 1.0 µm |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
- Image processing
Image processing
| Startup model | Type of model: NONE |
|---|---|
| Final reconstruction | Resolution.type: BY AUTHOR / Resolution: 3.66 Å / Resolution method: FSC 0.143 CUT-OFF / Number images used: 58843 |
| Initial angle assignment | Type: ANGULAR RECONSTITUTION |
| Final angle assignment | Type: ANGULAR RECONSTITUTION |
-Atomic model buiding 1
| Refinement | Space: REAL / Protocol: AB INITIO MODEL |
|---|---|
| Output model |  PDB-7f6v: |
 Movie
Movie Controller
Controller



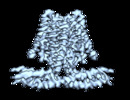








 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)





















