+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-30319 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | E30 F-particle in complex with CD55 | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | Echovirus 30 / mature / attachement receptor / VIRUS | |||||||||
| Function / homology |  Function and homology information Function and homology informationregulation of lipopolysaccharide-mediated signaling pathway / negative regulation of complement activation / regulation of complement-dependent cytotoxicity / regulation of complement activation / respiratory burst / positive regulation of CD4-positive, alpha-beta T cell activation / positive regulation of CD4-positive, alpha-beta T cell proliferation / Class B/2 (Secretin family receptors) / symbiont-mediated suppression of host cytoplasmic pattern recognition receptor signaling pathway via inhibition of RIG-I activity / ficolin-1-rich granule membrane ...regulation of lipopolysaccharide-mediated signaling pathway / negative regulation of complement activation / regulation of complement-dependent cytotoxicity / regulation of complement activation / respiratory burst / positive regulation of CD4-positive, alpha-beta T cell activation / positive regulation of CD4-positive, alpha-beta T cell proliferation / Class B/2 (Secretin family receptors) / symbiont-mediated suppression of host cytoplasmic pattern recognition receptor signaling pathway via inhibition of RIG-I activity / ficolin-1-rich granule membrane / complement activation, classical pathway / transport vesicle / side of membrane / COPI-mediated anterograde transport / endoplasmic reticulum-Golgi intermediate compartment membrane / secretory granule membrane / Regulation of Complement cascade / picornain 2A / symbiont-mediated suppression of host mRNA export from nucleus / symbiont genome entry into host cell via pore formation in plasma membrane / picornain 3C / T=pseudo3 icosahedral viral capsid / positive regulation of T cell cytokine production / host cell cytoplasmic vesicle membrane / ribonucleoside triphosphate phosphatase activity / viral capsid / nucleoside-triphosphate phosphatase / host cell / channel activity / positive regulation of cytosolic calcium ion concentration / virus receptor activity / monoatomic ion transmembrane transport / DNA replication / RNA helicase activity / membrane raft / endocytosis involved in viral entry into host cell / symbiont-mediated suppression of host gene expression / Golgi membrane / symbiont-mediated activation of host autophagy / innate immune response / RNA-directed RNA polymerase / cysteine-type endopeptidase activity / viral RNA genome replication / RNA-directed RNA polymerase activity / Neutrophil degranulation / DNA-templated transcription / lipid binding / virion attachment to host cell / host cell nucleus / structural molecule activity / cell surface / proteolysis / RNA binding / extracellular exosome / extracellular region / zinc ion binding / ATP binding / membrane / plasma membrane Similarity search - Function | |||||||||
| Biological species |  Echovirus E30 / Echovirus E30 /  Homo sapiens (human) Homo sapiens (human) | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 3.6 Å | |||||||||
 Authors Authors | Wang K / Zhu L | |||||||||
| Funding support |  China, 1 items China, 1 items
| |||||||||
 Citation Citation |  Journal: Nat Commun / Year: 2020 Journal: Nat Commun / Year: 2020Title: Structures of Echovirus 30 in complex with its receptors inform a rational prediction for enterovirus receptor usage. Authors: Kang Wang / Ling Zhu / Yao Sun / Minhao Li / Xin Zhao / Lunbiao Cui / Li Zhang / George F Gao / Weiwei Zhai / Fengcai Zhu / Zihe Rao / Xiangxi Wang /  Abstract: Receptor usage that determines cell tropism and drives viral classification closely correlates with the virus structure. Enterovirus B (EV-B) consists of several subgroups according to receptor ...Receptor usage that determines cell tropism and drives viral classification closely correlates with the virus structure. Enterovirus B (EV-B) consists of several subgroups according to receptor usage, among which echovirus 30 (E30), a leading causative agent for human aseptic meningitis, utilizes FcRn as an uncoating receptor. However, receptors for many EVs remain unknown. Here we analyzed the atomic structures of E30 mature virion, empty- and A-particles, which reveals serotype-specific epitopes and striking conformational differences between the subgroups within EV-Bs. Of these, the VP1 BC loop markedly distinguishes E30 from other EV-Bs, indicative of a role as a structural marker for EV-B. By obtaining cryo-electron microscopy structures of E30 in complex with its receptor FcRn and CD55 and comparing its homologs, we deciphered the underlying molecular basis for receptor recognition. Together with experimentally derived viral receptor identifications, we developed a structure-based in silico algorithm to inform a rational prediction for EV receptor usage. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_30319.map.gz emd_30319.map.gz | 96 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-30319-v30.xml emd-30319-v30.xml emd-30319.xml emd-30319.xml | 16.9 KB 16.9 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_30319.png emd_30319.png | 109.4 KB | ||
| Filedesc metadata |  emd-30319.cif.gz emd-30319.cif.gz | 6.6 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-30319 http://ftp.pdbj.org/pub/emdb/structures/EMD-30319 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-30319 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-30319 | HTTPS FTP |
-Related structure data
| Related structure data |  7c9wMC  7c9sC  7c9tC  7c9uC  7c9vC  7c9xC  7c9yC  7c9zC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_30319.map.gz / Format: CCP4 / Size: 103 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_30319.map.gz / Format: CCP4 / Size: 103 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.314 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
- Sample components
Sample components
+Entire : E30 F-particle in complex with CD55
+Supramolecule #1: E30 F-particle in complex with CD55
+Supramolecule #2: E30 F-particle in complex with FcRn
+Supramolecule #3: CD55 or DAF (decay-accelerating factor)
+Macromolecule #1: VP1
+Macromolecule #2: VP2
+Macromolecule #3: VP3
+Macromolecule #4: VP4
+Macromolecule #5: Complement decay-accelerating factor
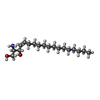
+Macromolecule #6: SPHINGOSINE
+Macromolecule #7: MYRISTIC ACID
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.4 |
|---|---|
| Grid | Model: Quantifoil R1.2/1.3 / Material: GOLD / Support film - Material: CARBON / Support film - topology: HOLEY / Pretreatment - Type: PLASMA CLEANING / Pretreatment - Time: 60 sec. |
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Instrument: FEI VITROBOT MARK I |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Detector mode: SUPER-RESOLUTION / Average electron dose: 30.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: DARK FIELD / Nominal defocus max: 2.5 µm / Nominal defocus min: 1.2 µm |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
- Image processing
Image processing
| Startup model | Type of model: INSILICO MODEL |
|---|---|
| Final reconstruction | Resolution.type: BY AUTHOR / Resolution: 3.6 Å / Resolution method: FSC 0.143 CUT-OFF / Software - Name: RELION (ver. 3.0) / Number images used: 1016 |
| Initial angle assignment | Type: PROJECTION MATCHING |
| Final angle assignment | Type: PROJECTION MATCHING / Software - Name: RELION |
-Atomic model buiding 1
| Refinement | Space: REAL / Protocol: RIGID BODY FIT |
|---|---|
| Output model |  PDB-7c9w: |
 Movie
Movie Controller
Controller




























 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)