[English] 日本語
 Yorodumi
Yorodumi- EMDB-30249: Cryo-EM structure of the formoterol-bound beta2 adrenergic recept... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-30249 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo-EM structure of the formoterol-bound beta2 adrenergic receptor-Gs protein complex. | |||||||||
 Map data Map data | A cryo-EM structure of formoterol-bound beta2 adrenergic recepter in complex with Gs protein at 3.82 angstroms. | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | Receptor / Formoterol / Complex / SIGNALING PROTEIN | |||||||||
| Function / homology |  Function and homology information Function and homology informationOlfactory Signaling Pathway / Sensory perception of sweet, bitter, and umami (glutamate) taste / Synthesis, secretion, and inactivation of Glucagon-like Peptide-1 (GLP-1) / Activation of the phototransduction cascade / positive regulation of mini excitatory postsynaptic potential / beta2-adrenergic receptor activity / negative regulation of smooth muscle contraction / AMPA selective glutamate receptor signaling pathway / norepinephrine-epinephrine-mediated vasodilation involved in regulation of systemic arterial blood pressure / positive regulation of autophagosome maturation ...Olfactory Signaling Pathway / Sensory perception of sweet, bitter, and umami (glutamate) taste / Synthesis, secretion, and inactivation of Glucagon-like Peptide-1 (GLP-1) / Activation of the phototransduction cascade / positive regulation of mini excitatory postsynaptic potential / beta2-adrenergic receptor activity / negative regulation of smooth muscle contraction / AMPA selective glutamate receptor signaling pathway / norepinephrine-epinephrine-mediated vasodilation involved in regulation of systemic arterial blood pressure / positive regulation of autophagosome maturation / heat generation / norepinephrine binding / Adrenoceptors / positive regulation of lipophagy / Activation of G protein gated Potassium channels / G-protein activation / G beta:gamma signalling through PI3Kgamma / Prostacyclin signalling through prostacyclin receptor / G beta:gamma signalling through PLC beta / ADP signalling through P2Y purinoceptor 1 / Thromboxane signalling through TP receptor / Presynaptic function of Kainate receptors / G beta:gamma signalling through CDC42 / Inhibition of voltage gated Ca2+ channels via Gbeta/gamma subunits / G alpha (12/13) signalling events / Glucagon-type ligand receptors / G beta:gamma signalling through BTK / ADP signalling through P2Y purinoceptor 12 / Adrenaline,noradrenaline inhibits insulin secretion / negative regulation of G protein-coupled receptor signaling pathway / negative regulation of multicellular organism growth / Cooperation of PDCL (PhLP1) and TRiC/CCT in G-protein beta folding / Ca2+ pathway / Thrombin signalling through proteinase activated receptors (PARs) / G alpha (z) signalling events / Extra-nuclear estrogen signaling / G alpha (s) signalling events / G alpha (q) signalling events / response to psychosocial stress / adrenergic receptor signaling pathway / endosome to lysosome transport / G alpha (i) signalling events / Glucagon-like Peptide-1 (GLP1) regulates insulin secretion / diet induced thermogenesis / High laminar flow shear stress activates signaling by PIEZO1 and PECAM1:CDH5:KDR in endothelial cells / Vasopressin regulates renal water homeostasis via Aquaporins / positive regulation of cAMP/PKA signal transduction / adenylate cyclase binding / smooth muscle contraction / PKA activation in glucagon signalling / developmental growth / hair follicle placode formation / potassium channel regulator activity / bone resorption / positive regulation of bone mineralization / intracellular transport / D1 dopamine receptor binding / neuronal dense core vesicle / vascular endothelial cell response to laminar fluid shear stress / renal water homeostasis / activation of adenylate cyclase activity / Hedgehog 'off' state / intercellular bridge / regulation of sodium ion transport / adenylate cyclase-activating adrenergic receptor signaling pathway / brown fat cell differentiation / regulation of insulin secretion / cellular response to glucagon stimulus / receptor-mediated endocytosis / response to cold / adenylate cyclase activator activity / trans-Golgi network membrane / negative regulation of inflammatory response to antigenic stimulus / clathrin-coated endocytic vesicle membrane / bone development / platelet aggregation / cognition / adenylate cyclase-modulating G protein-coupled receptor signaling pathway / G-protein beta/gamma-subunit complex binding / cellular response to amyloid-beta / adenylate cyclase-activating G protein-coupled receptor signaling pathway / Prostacyclin signalling through prostacyclin receptor / Glucagon signaling in metabolic regulation / mitotic spindle / photoreceptor disc membrane / Glucagon-type ligand receptors / sensory perception of smell / Vasopressin regulates renal water homeostasis via Aquaporins / Glucagon-like Peptide-1 (GLP1) regulates insulin secretion / G alpha (z) signalling events / ADORA2B mediated anti-inflammatory cytokines production / cellular response to catecholamine stimulus / adenylate cyclase-activating dopamine receptor signaling pathway / Cargo recognition for clathrin-mediated endocytosis / GPER1 signaling / G-protein beta-subunit binding / cellular response to prostaglandin E stimulus / heterotrimeric G-protein complex / sensory perception of taste / positive regulation of cold-induced thermogenesis Similarity search - Function | |||||||||
| Biological species |  Homo sapiens (human) / Homo sapiens (human) /   | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 3.82 Å | |||||||||
 Authors Authors | Zhang YN / Yang F | |||||||||
 Citation Citation |  Journal: Cell Discov / Year: 2020 Journal: Cell Discov / Year: 2020Title: Single-particle cryo-EM structural studies of the βAR-Gs complex bound with a full agonist formoterol. Authors: Yanan Zhang / Fan Yang / Shenglong Ling / Pei Lv / Yingxin Zhou / Wei Fang / Wenjing Sun / Longhua Zhang / Pan Shi / Changlin Tian /  | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_30249.map.gz emd_30249.map.gz | 2.1 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-30249-v30.xml emd-30249-v30.xml emd-30249.xml emd-30249.xml | 15.2 KB 15.2 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_30249.png emd_30249.png | 57.1 KB | ||
| Filedesc metadata |  emd-30249.cif.gz emd-30249.cif.gz | 6.3 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-30249 http://ftp.pdbj.org/pub/emdb/structures/EMD-30249 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-30249 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-30249 | HTTPS FTP |
-Related structure data
| Related structure data |  7bz2MC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_30249.map.gz / Format: CCP4 / Size: 23.8 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_30249.map.gz / Format: CCP4 / Size: 23.8 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | A cryo-EM structure of formoterol-bound beta2 adrenergic recepter in complex with Gs protein at 3.82 angstroms. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.06 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
- Sample components
Sample components
-Entire : Beta2 adrenergic receptor-Gs trimer complex with formoterol
| Entire | Name: Beta2 adrenergic receptor-Gs trimer complex with formoterol |
|---|---|
| Components |
|
-Supramolecule #1: Beta2 adrenergic receptor-Gs trimer complex with formoterol
| Supramolecule | Name: Beta2 adrenergic receptor-Gs trimer complex with formoterol type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1-#5 |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 151.40 kDa/nm |
-Macromolecule #1: Guanine nucleotide-binding protein G(s) subunit alpha isoforms short
| Macromolecule | Name: Guanine nucleotide-binding protein G(s) subunit alpha isoforms short type: protein_or_peptide / ID: 1 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 45.812594 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: SMGCLGNSKT EDQRNEEKAQ REANKKIEKQ LQKDKQVYRA THRLLLLGAG ESGKSTIVKQ MRILHVNGFN GEGGEEDPQA ARSNSDGEK ATKVQDIKNN LKEAIETIVA AMSNLVPPVE LANPENQFRV DYILSVMNVP DFDFPPEFYE HAKALWEDEG V RACYERSN ...String: SMGCLGNSKT EDQRNEEKAQ REANKKIEKQ LQKDKQVYRA THRLLLLGAG ESGKSTIVKQ MRILHVNGFN GEGGEEDPQA ARSNSDGEK ATKVQDIKNN LKEAIETIVA AMSNLVPPVE LANPENQFRV DYILSVMNVP DFDFPPEFYE HAKALWEDEG V RACYERSN EYQLIDCAQY FLDKIDVIKQ ADYVPSDQDL LRCRVLTSGI FETKFQVDKV NFHMFDVGGQ RDERRKWIQC FN DVTAIIF VVASSSYNMV IREDNQTNRL QEALNLFKSI WNNRWLRTIS VILFLNKQDL LAEKVLAGKS KIEDYFPEFA RYT TPEDAT PEPGEDPRVT RAKYFIRDEF LRISTASGDG RHYCYPHFTC AVDTENIRRV FNDCRDIIQR MHLRQYELL UniProtKB: Guanine nucleotide-binding protein G(s) subunit alpha isoforms short |
-Macromolecule #2: Guanine nucleotide-binding protein G(I)/G(S)/G(T) subunit beta-1
| Macromolecule | Name: Guanine nucleotide-binding protein G(I)/G(S)/G(T) subunit beta-1 type: protein_or_peptide / ID: 2 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 37.346812 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MSELDQLRQE AEQLKNQIRD ARKACADATL SQITNNIDPV GRIQMRTRRT LRGHLAKIYA MHWGTDSRLL VSASQDGKLI IWDSYTTNK VHAIPLRSSW VMTCAYAPSG NYVACGGLDN ICSIYNLKTR EGNVRVSREL AGHTGYLSCC RFLDDNQIVT S SGDTTCAL ...String: MSELDQLRQE AEQLKNQIRD ARKACADATL SQITNNIDPV GRIQMRTRRT LRGHLAKIYA MHWGTDSRLL VSASQDGKLI IWDSYTTNK VHAIPLRSSW VMTCAYAPSG NYVACGGLDN ICSIYNLKTR EGNVRVSREL AGHTGYLSCC RFLDDNQIVT S SGDTTCAL WDIETGQQTT TFTGHTGDVM SLSLAPDTRL FVSGACDASA KLWDVREGMC RQTFTGHESD INAICFFPNG NA FATGSDD ATCRLFDLRA DQELMTYSHD NIICGITSVS FSKSGRLLLA GYDDFNCNVW DALKADSAGV LAGHDNRVSC LGV TDDGMA VATGSWDSFL KIWN UniProtKB: Guanine nucleotide-binding protein G(I)/G(S)/G(T) subunit beta-1 |
-Macromolecule #3: Guanine nucleotide-binding protein G(I)/G(S)/G(O) subunit gamma-2
| Macromolecule | Name: Guanine nucleotide-binding protein G(I)/G(S)/G(O) subunit gamma-2 type: protein_or_peptide / ID: 3 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 8.805155 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MHHHHHHMAS NNTASIAQAR KLVEQLKMEA NIDRIKVSKA AADLMAYCEA HAKEDPLLTP VPASENPFRE KKFFSAIL UniProtKB: Guanine nucleotide-binding protein G(I)/G(S)/G(O) subunit gamma-2 |
-Macromolecule #4: Nb35 nanobody
| Macromolecule | Name: Nb35 nanobody / type: protein_or_peptide / ID: 4 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 17.483693 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MKYLLPTAAA GLLLLAAQPA MAMQVQLQES GGGLVQPGGS LRLSCAASGF TFSNYKMNWV RQAPGKGLEW VSDISQSGAS ISYTGSVKG RFTISRDNAK NTLYLQMNSL KPEDTAVYYC ARCPAPFTRD CFDVTSTTYA YRGQGTQVTV SSHHHHHHEP E A |
-Macromolecule #5: Beta2 adrenergic receptor
| Macromolecule | Name: Beta2 adrenergic receptor / type: protein_or_peptide / ID: 5 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 42.010844 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MKTIIALSYI FSLVFADYKD DDDAMGQPGN GSAFLLAPNR SHAPDHDVTQ QRDEVWVVGM GIVMSLIVLA IVFGNVLVIT AIAKFERLQ TVTNYFITSL AVADLVMGLA VVPFGAAHIL TKTWTFGNFW CEFWTSIDVL CVTASIWTLV VIAVDRYFAI T SPFKYQSL ...String: MKTIIALSYI FSLVFADYKD DDDAMGQPGN GSAFLLAPNR SHAPDHDVTQ QRDEVWVVGM GIVMSLIVLA IVFGNVLVIT AIAKFERLQ TVTNYFITSL AVADLVMGLA VVPFGAAHIL TKTWTFGNFW CEFWTSIDVL CVTASIWTLV VIAVDRYFAI T SPFKYQSL LTKNKARVII LMVWIVSGLT SFLPIQMHWY RATHQEAINC YAEETCCDFF TNQAYAIASS IVSFYVPLVI MV FVYSRVF QEAKRQLQKI DKSEGRFHVQ NRSSKFALKE HKALKTLGII MGTFTLAWLP FFIVNIVHVI QDNLIRKEVY ILL NWIGYV NSGFNPLIYS RSPDFRIAFQ ELLALRRSSL KHHHHHHHHH H |
-Macromolecule #6: ~{N}-[5-[(1~{R})-2-[[(2~{R})-1-(4-methoxyphenyl)propan-2-yl]amino...
| Macromolecule | Name: ~{N}-[5-[(1~{R})-2-[[(2~{R})-1-(4-methoxyphenyl)propan-2-yl]amino]-1-oxidanyl-ethyl]-2-oxidanyl-phenyl]methanamide type: ligand / ID: 6 / Number of copies: 1 / Formula: H98 |
|---|---|
| Molecular weight | Theoretical: 344.405 Da |
| Chemical component information | 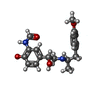 ChemComp-H98: |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 0.5 mg/mL |
|---|---|
| Buffer | pH: 7.5 |
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 4 K |
| Details | This sample was monodisperse. |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Detector mode: COUNTING / Number real images: 1625 / Average electron dose: 58.7 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: SPOT SCAN / Imaging mode: DARK FIELD / Nominal magnification: 29000 |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
- Image processing
Image processing
| Startup model | Type of model: NONE |
|---|---|
| Final reconstruction | Resolution.type: BY AUTHOR / Resolution: 3.82 Å / Resolution method: FSC 0.143 CUT-OFF / Number images used: 219254 |
| Initial angle assignment | Type: PROJECTION MATCHING |
| Final angle assignment | Type: PROJECTION MATCHING |
 Movie
Movie Controller
Controller



























 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)





















