[English] 日本語
 Yorodumi
Yorodumi- EMDB-29920: Cardiac amyloid fibrils extracted from a wild-type ATTR amyloidos... -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cardiac amyloid fibrils extracted from a wild-type ATTR amyloidosis patient | |||||||||
 Map data Map data | Main map | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | Transthyretin / Amyloidosis / Systemic amyloidosis / ATTR / Cardiac / PROTEIN FIBRIL | |||||||||
| Function / homology |  Function and homology information Function and homology informationDefective visual phototransduction due to STRA6 loss of function / negative regulation of glomerular filtration / The canonical retinoid cycle in rods (twilight vision) / hormone binding / purine nucleobase metabolic process / molecular sequestering activity / Non-integrin membrane-ECM interactions / phototransduction, visible light / retinoid metabolic process / Retinoid metabolism and transport ...Defective visual phototransduction due to STRA6 loss of function / negative regulation of glomerular filtration / The canonical retinoid cycle in rods (twilight vision) / hormone binding / purine nucleobase metabolic process / molecular sequestering activity / Non-integrin membrane-ECM interactions / phototransduction, visible light / retinoid metabolic process / Retinoid metabolism and transport / hormone activity / azurophil granule lumen / Amyloid fiber formation / Neutrophil degranulation / protein-containing complex binding / protein-containing complex / extracellular space / extracellular exosome / extracellular region / identical protein binding Similarity search - Function | |||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | |||||||||
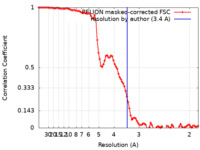
| Method | helical reconstruction / cryo EM / Resolution: 3.4 Å | |||||||||
 Authors Authors | Nguyen BA / Saelices L | |||||||||
| Funding support |  United States, 2 items United States, 2 items
| |||||||||
 Citation Citation |  Journal: Commun Biol / Year: 2024 Journal: Commun Biol / Year: 2024Title: Cryo-EM confirms a common fibril fold in the heart of four patients with ATTRwt amyloidosis. Authors: Binh An Nguyen / Virender Singh / Shumaila Afrin / Preeti Singh / Maja Pekala / Yasmin Ahmed / Rose Pedretti / Jacob Canepa / Andrew Lemoff / Barbara Kluve-Beckerman / Pawel M Wydorski / ...Authors: Binh An Nguyen / Virender Singh / Shumaila Afrin / Preeti Singh / Maja Pekala / Yasmin Ahmed / Rose Pedretti / Jacob Canepa / Andrew Lemoff / Barbara Kluve-Beckerman / Pawel M Wydorski / Farzeen Chhapra / Lorena Saelices /  Abstract: ATTR amyloidosis results from the conversion of transthyretin into amyloid fibrils that deposit in tissues causing organ failure and death. This conversion is facilitated by mutations in ATTRv ...ATTR amyloidosis results from the conversion of transthyretin into amyloid fibrils that deposit in tissues causing organ failure and death. This conversion is facilitated by mutations in ATTRv amyloidosis, or aging in ATTRwt amyloidosis. ATTRv amyloidosis exhibits extreme phenotypic variability, whereas ATTRwt amyloidosis presentation is consistent and predictable. Previously, we found unique structural variabilities in cardiac amyloid fibrils from polyneuropathic ATTRv-I84S patients. In contrast, cardiac fibrils from five genotypically different patients with cardiomyopathy or mixed phenotypes are structurally homogeneous. To understand fibril structure's impact on phenotype, it is necessary to study the fibrils from multiple patients sharing genotype and phenotype. Here we show the cryo-electron microscopy structures of fibrils extracted from four cardiomyopathic ATTRwt amyloidosis patients. Our study confirms that they share identical conformations with minimal structural variability, consistent with their homogenous clinical presentation. Our study contributes to the understanding of ATTR amyloidosis biopathology and calls for further studies. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_29920.map.gz emd_29920.map.gz | 49.2 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-29920-v30.xml emd-29920-v30.xml emd-29920.xml emd-29920.xml | 19.6 KB 19.6 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_29920_fsc.xml emd_29920_fsc.xml | 9.1 KB | Display |  FSC data file FSC data file |
| Images |  emd_29920.png emd_29920.png | 85.6 KB | ||
| Filedesc metadata |  emd-29920.cif.gz emd-29920.cif.gz | 5.7 KB | ||
| Others |  emd_29920_additional_1.map.gz emd_29920_additional_1.map.gz emd_29920_half_map_1.map.gz emd_29920_half_map_1.map.gz emd_29920_half_map_2.map.gz emd_29920_half_map_2.map.gz | 5.5 MB 49.5 MB 49.6 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-29920 http://ftp.pdbj.org/pub/emdb/structures/EMD-29920 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-29920 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-29920 | HTTPS FTP |
-Validation report
| Summary document |  emd_29920_validation.pdf.gz emd_29920_validation.pdf.gz | 774.7 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_29920_full_validation.pdf.gz emd_29920_full_validation.pdf.gz | 774.3 KB | Display | |
| Data in XML |  emd_29920_validation.xml.gz emd_29920_validation.xml.gz | 16.3 KB | Display | |
| Data in CIF |  emd_29920_validation.cif.gz emd_29920_validation.cif.gz | 21.5 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-29920 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-29920 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-29920 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-29920 | HTTPS FTP |
-Related structure data
| Related structure data |  8gbrMC  8g9rC C: citing same article ( M: atomic model generated by this map |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_29920.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_29920.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Main map | ||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.946 Å | ||||||||||||||||||||||||||||||||||||
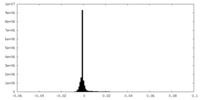
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Additional map: post-processed map
| File | emd_29920_additional_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | post-processed map | ||||||||||||
| Projections & Slices |
| ||||||||||||
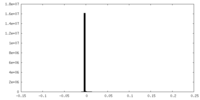
| Density Histograms |
-Half map: half-map 1
| File | emd_29920_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | half-map 1 | ||||||||||||
| Projections & Slices |
| ||||||||||||
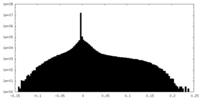
| Density Histograms |
-Half map: half-map 2
| File | emd_29920_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | half-map 2 | ||||||||||||
| Projections & Slices |
| ||||||||||||
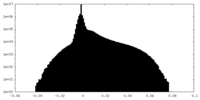
| Density Histograms |
- Sample components
Sample components
-Entire : cardiac amyloid fibril of wild-type transthyretin amyloidosis
| Entire | Name: cardiac amyloid fibril of wild-type transthyretin amyloidosis |
|---|---|
| Components |
|
-Supramolecule #1: cardiac amyloid fibril of wild-type transthyretin amyloidosis
| Supramolecule | Name: cardiac amyloid fibril of wild-type transthyretin amyloidosis type: tissue / ID: 1 / Parent: 0 / Macromolecule list: all |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) / Organ: Heart / Tissue: Cardiac Homo sapiens (human) / Organ: Heart / Tissue: Cardiac |
-Macromolecule #1: Transthyretin
| Macromolecule | Name: Transthyretin / type: protein_or_peptide / ID: 1 / Number of copies: 5 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) / Organ: Heart / Tissue: Cardiac Homo sapiens (human) / Organ: Heart / Tissue: Cardiac |
| Molecular weight | Theoretical: 15.904984 KDa |
| Sequence | String: MASHRLLLLC LAGLVFVSEA GPTGTGESKC PLMVKVLDAV RGSPAINVAV HVFRKAADDT WEPFASGKTS ESGELHGLTT EEEFVEGIY KVEIDTKSYW KALGISPFHE HAEVVFTAND SGPRRYTIAA LLSPYSYSTT AVVTNPKE UniProtKB: Transthyretin |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | helical reconstruction |
| Aggregation state | filament |
- Sample preparation
Sample preparation
| Buffer | pH: 7 / Details: fibrils are in water |
|---|---|
| Grid | Model: Quantifoil R1.2/1.3 / Material: COPPER / Mesh: 300 / Support film - Material: CARBON / Support film - topology: HOLEY / Support film - Film thickness: 12 / Pretreatment - Type: GLOW DISCHARGE / Pretreatment - Time: 30 sec. / Pretreatment - Atmosphere: AIR |
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 295.15 K / Instrument: FEI VITROBOT MARK IV |
| Details | Purified by water extraction |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Number grids imaged: 1 / Number real images: 8243 / Average exposure time: 4.98 sec. / Average electron dose: 40.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm / Nominal defocus max: 2.3000000000000003 µm / Nominal defocus min: 1.7 µm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
- Image processing
Image processing
-Atomic model buiding 1
| Initial model | PDB ID: Chain - Chain ID: A / Chain - Source name: PDB / Chain - Initial model type: experimental model / Details: Similar structure |
|---|---|
| Details | Initial fitting was done using Coot with rigid body fit, then real space refinement for better fitting |
| Refinement | Space: REAL / Protocol: RIGID BODY FIT / Overall B value: 71.63 / Target criteria: Cross-correlation coefficient |
| Output model |  PDB-8gbr: |
 Movie
Movie Controller
Controller











 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)