[English] 日本語
 Yorodumi
Yorodumi- EMDB-29620: Cryo-EM structure of an E. coli non-rotated ribosome termination ... -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo-EM structure of an E. coli non-rotated ribosome termination complex bound with apoRF3, RF1, P- and E-site tRNAPhe (Composite state I-B) | ||||||||||||
 Map data Map data | Cryo-EM structure of an E. coli non-rotated ribosome termination complex bound with apoRF3, RF1, P- and E-site tRNAPhe (Composite state I-B) | ||||||||||||
 Sample Sample |
| ||||||||||||
 Keywords Keywords | release factor 1 / release factor 3 / termination complex / cryo-EM / tRNA / RIBOSOME | ||||||||||||
| Function / homology |  Function and homology information Function and homology informationregulation of translational termination / translation release factor activity, codon nonspecific / translation release factor activity, codon specific / guanosine tetraphosphate binding / negative regulation of cytoplasmic translational initiation / stringent response / positive regulation of ribosome biogenesis / translational termination / DnaA-L2 complex / negative regulation of translational initiation ...regulation of translational termination / translation release factor activity, codon nonspecific / translation release factor activity, codon specific / guanosine tetraphosphate binding / negative regulation of cytoplasmic translational initiation / stringent response / positive regulation of ribosome biogenesis / translational termination / DnaA-L2 complex / negative regulation of translational initiation / negative regulation of DNA-templated DNA replication initiation / mRNA regulatory element binding translation repressor activity / assembly of large subunit precursor of preribosome / cytosolic ribosome assembly / ribosome assembly / transcription antitermination / regulation of cell growth / translational initiation / DNA-templated transcription termination / maintenance of translational fidelity / mRNA 5'-UTR binding / GDP binding / large ribosomal subunit / transferase activity / ribosome binding / ribosomal small subunit biogenesis / ribosomal small subunit assembly / 5S rRNA binding / ribosomal large subunit assembly / small ribosomal subunit / small ribosomal subunit rRNA binding / large ribosomal subunit rRNA binding / cytosolic small ribosomal subunit / cytosolic large ribosomal subunit / cytoplasmic translation / tRNA binding / negative regulation of translation / rRNA binding / structural constituent of ribosome / ribosome / translation / ribonucleoprotein complex / response to antibiotic / mRNA binding / GTPase activity / GTP binding / RNA binding / zinc ion binding / membrane / cytosol / cytoplasm Similarity search - Function | ||||||||||||
| Biological species |   Escherichia phage T4 (virus) / Escherichia phage T4 (virus) /  | ||||||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 3.1 Å | ||||||||||||
 Authors Authors | Rybak MY / Li L / Lin J / Gagnon MG | ||||||||||||
| Funding support |  United States, United States,  China, 3 items China, 3 items
| ||||||||||||
 Citation Citation |  Journal: Nat Struct Mol Biol / Year: 2024 Journal: Nat Struct Mol Biol / Year: 2024Title: The ribosome termination complex remodels release factor RF3 and ejects GDP. Authors: Li Li / Mariia Yu Rybak / Jinzhong Lin / Matthieu G Gagnon /   Abstract: Translation termination involves release factors RF1, RF2 and the GTPase RF3 that recycles RF1 and RF2 from the ribosome. RF3 dissociates from the ribosome in the GDP-bound form and must then ...Translation termination involves release factors RF1, RF2 and the GTPase RF3 that recycles RF1 and RF2 from the ribosome. RF3 dissociates from the ribosome in the GDP-bound form and must then exchange GDP for GTP. The 70S ribosome termination complex (70S-TC) accelerates GDP exchange in RF3, suggesting that the 70S-TC can function as the guanine nucleotide exchange factor for RF3. Here, we use cryogenic-electron microscopy to elucidate the mechanism of GDP dissociation from RF3 catalyzed by the Escherichia coli 70S-TC. The non-rotated ribosome bound to RF1 remodels RF3 and induces a peptide flip in the phosphate-binding loop, efficiently ejecting GDP. Binding of GTP allows RF3 to dock at the GTPase center, promoting the dissociation of RF1 from the ribosome. The structures recapitulate the functional cycle of RF3 on the ribosome and uncover the mechanism by which the 70S-TC allosterically dismantles the phosphate-binding groove in RF3, a previously overlooked function of the ribosome. | ||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_29620.map.gz emd_29620.map.gz | 434.7 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-29620-v30.xml emd-29620-v30.xml emd-29620.xml emd-29620.xml | 86.4 KB 86.4 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_29620.png emd_29620.png | 171.6 KB | ||
| Filedesc metadata |  emd-29620.cif.gz emd-29620.cif.gz | 17.1 KB | ||
| Others |  emd_29620_half_map_1.map.gz emd_29620_half_map_1.map.gz emd_29620_half_map_2.map.gz emd_29620_half_map_2.map.gz | 434.5 MB 434.5 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-29620 http://ftp.pdbj.org/pub/emdb/structures/EMD-29620 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-29620 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-29620 | HTTPS FTP |
-Related structure data
| Related structure data |  8fzdMC  8fzeC  8fzfC  8fzgC  8fzhC  8fziC  8fzjC C: citing same article ( M: atomic model generated by this map |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_29620.map.gz / Format: CCP4 / Size: 512 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_29620.map.gz / Format: CCP4 / Size: 512 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Cryo-EM structure of an E. coli non-rotated ribosome termination complex bound with apoRF3, RF1, P- and E-site tRNAPhe (Composite state I-B) | ||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.85 Å | ||||||||||||||||||||||||||||||||||||
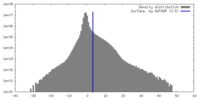
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Half map: Cryo-EM structure of an E. coli non-rotated ribosome...
| File | emd_29620_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Cryo-EM structure of an E. coli non-rotated ribosome termination complex bound with apoRF3, RF1, P- and E-site tRNAPhe (Composite state I-B). Half map. | ||||||||||||
| Projections & Slices |
| ||||||||||||
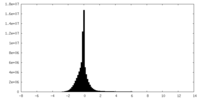
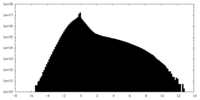
| Density Histograms |
-Half map: Cryo-EM structure of an E. coli non-rotated ribosome...
| File | emd_29620_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Cryo-EM structure of an E. coli non-rotated ribosome termination complex bound with apoRF3, RF1, P- and E-site tRNAPhe (Composite state I-B). Half map. | ||||||||||||
| Projections & Slices |
| ||||||||||||
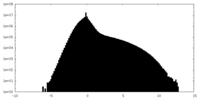
| Density Histograms |
- Sample components
Sample components
+Entire : Cryo-EM structure of an E. coli non-rotated ribosome termination ...
+Supramolecule #1: Cryo-EM structure of an E. coli non-rotated ribosome termination ...
+Macromolecule #1: 16S Ribosomal RNA
+Macromolecule #22: P-site phenylalanyl-tRNA
+Macromolecule #23: P-site phenylalanyl-tRNA
+Macromolecule #24: F-UAA mRNA
+Macromolecule #27: 23S Ribosomal RNA
+Macromolecule #28: 5S Ribosomal RNA
+Macromolecule #2: 30S ribosomal protein S2
+Macromolecule #3: 30S ribosomal protein S3
+Macromolecule #4: 30S ribosomal protein S4
+Macromolecule #5: 30S ribosomal protein S5
+Macromolecule #6: 30S ribosomal protein S6
+Macromolecule #7: 30S ribosomal protein S7
+Macromolecule #8: 30S ribosomal protein S8
+Macromolecule #9: 30S ribosomal protein S9
+Macromolecule #10: 30S ribosomal protein S10
+Macromolecule #11: 30S ribosomal protein S11
+Macromolecule #12: 30S ribosomal protein S12
+Macromolecule #13: 30S ribosomal protein S13
+Macromolecule #14: 30S ribosomal protein S14
+Macromolecule #15: 30S ribosomal protein S15
+Macromolecule #16: 30S ribosomal protein S16
+Macromolecule #17: 30S ribosomal protein S17
+Macromolecule #18: 30S ribosomal protein S18
+Macromolecule #19: 30S ribosomal protein S19
+Macromolecule #20: 30S ribosomal protein S20
+Macromolecule #21: 30S ribosomal protein S21
+Macromolecule #25: Peptide chain release factor 1
+Macromolecule #26: Peptide chain release factor RF3
+Macromolecule #29: 50S ribosomal protein L2
+Macromolecule #30: 50S ribosomal protein L3
+Macromolecule #31: 50S ribosomal protein L4
+Macromolecule #32: 50S ribosomal protein L5
+Macromolecule #33: 50S ribosomal protein L6
+Macromolecule #34: 50S ribosomal protein L9
+Macromolecule #35: 50S ribosomal protein L10
+Macromolecule #36: 50S ribosomal protein L11
+Macromolecule #37: 50S ribosomal protein L13
+Macromolecule #38: 50S ribosomal protein L14
+Macromolecule #39: 50S ribosomal protein L15
+Macromolecule #40: 50S ribosomal protein L16
+Macromolecule #41: 50S ribosomal protein L17
+Macromolecule #42: 50S ribosomal protein L18
+Macromolecule #43: 50S ribosomal protein L19
+Macromolecule #44: 50S ribosomal protein L20
+Macromolecule #45: Ribosomal protein L21
+Macromolecule #46: 50S ribosomal protein L22
+Macromolecule #47: 50S ribosomal protein L23
+Macromolecule #48: 50S ribosomal protein L24
+Macromolecule #49: 50S ribosomal protein L25
+Macromolecule #50: 50S ribosomal protein L27
+Macromolecule #51: 50S ribosomal protein L28
+Macromolecule #52: 50S ribosomal protein L29
+Macromolecule #53: 50S ribosomal protein L30
+Macromolecule #54: 50S ribosomal protein L31
+Macromolecule #55: 50S ribosomal protein L32
+Macromolecule #56: 50S ribosomal protein L33
+Macromolecule #57: 50S ribosomal protein L34
+Macromolecule #58: 50S ribosomal protein L35
+Macromolecule #59: 50S ribosomal protein L36
+Macromolecule #60: MAGNESIUM ION
+Macromolecule #61: ZINC ION
+Macromolecule #62: water
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.4 Details: 5 mM Tris-HCl, 60 mM NH4Cl, 10 mM MgCl2, 6 mM B-mercaptoethanol |
|---|---|
| Grid | Model: Quantifoil R2/1 / Material: GOLD / Mesh: 200 / Support film - Material: GOLD / Support film - topology: HOLEY / Pretreatment - Type: PLASMA CLEANING / Pretreatment - Time: 30 sec. / Pretreatment - Atmosphere: OTHER |
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 85 % / Chamber temperature: 295 K / Instrument: LEICA EM GP |
- Electron microscopy
Electron microscopy
| Microscope | TFS KRIOS |
|---|---|
| Software | Name: EPU (ver. 2.11.1) |
| Image recording | Film or detector model: FEI FALCON III (4k x 4k) / Detector mode: INTEGRATING / Number grids imaged: 1 / Number real images: 10284 / Average exposure time: 1.0 sec. / Average electron dose: 40.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm / Nominal defocus max: 2.5 µm / Nominal defocus min: 1.0 µm / Nominal magnification: 96000 |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
+ Image processing
Image processing
-Atomic model buiding 1
| Initial model | PDB ID: Chain - Source name: PDB / Chain - Initial model type: experimental model |
|---|---|
| Software | Name:  UCSF Chimera (ver. 1.14) UCSF Chimera (ver. 1.14) |
| Refinement | Space: REAL / Protocol: RIGID BODY FIT |
| Output model |  PDB-8fzd: |
 Movie
Movie Controller
Controller
























 X (Sec.)
X (Sec.) Y (Row.)
Y (Row.) Z (Col.)
Z (Col.)