+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | V. radiata respiratory supercomplex I+III2 bridgeless class 4 | |||||||||
 Map data Map data | Bridgeless supercomplex I III2 class 4_mask | |||||||||
 Sample Sample |
| |||||||||
| Biological species |  Vigna radiata (mung bean) Vigna radiata (mung bean) | |||||||||
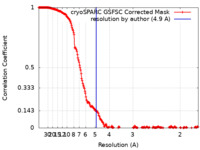
| Method | single particle reconstruction / cryo EM / Resolution: 4.9 Å | |||||||||
 Authors Authors | Maldonado M / Letts JA | |||||||||
| Funding support |  United States, 1 items United States, 1 items
| |||||||||
 Citation Citation |  Journal: Nat Plants / Year: 2023 Journal: Nat Plants / Year: 2023Title: Plant-specific features of respiratory supercomplex I + III from Vigna radiata. Authors: M Maldonado / Z Fan / K M Abe / J A Letts /  Abstract: The last steps of cellular respiration-an essential metabolic process in plants-are carried out by mitochondrial oxidative phosphorylation. This process involves a chain of multi-subunit membrane ...The last steps of cellular respiration-an essential metabolic process in plants-are carried out by mitochondrial oxidative phosphorylation. This process involves a chain of multi-subunit membrane protein complexes (complexes I-V) that form higher-order assemblies called supercomplexes. Although supercomplexes are the most physiologically relevant form of the oxidative phosphorylation complexes, their functions and structures remain mostly unknown. Here we present the cryogenic electron microscopy structure of the supercomplex I + III from Vigna radiata (mung bean). The structure contains the full subunit complement of complex I, including a newly assigned, plant-specific subunit. It also shows differences in the mitochondrial processing peptidase domain of complex III relative to a previously determined supercomplex with complex IV. The supercomplex interface, while reminiscent of that in other organisms, is plant specific, with a major interface involving complex III's mitochondrial processing peptidase domain and no participation of complex I's bridge domain. The complex I structure suggests that the bridge domain sets the angle between the enzyme's two arms, limiting large-scale conformational changes. Moreover, complex I's catalytic loops and its response in active-to-deactive assays suggest that, in V. radiata, the resting complex adopts a non-canonical state and can sample deactive- or open-like conformations even in the presence of substrate. This study widens our understanding of the possible conformations and behaviour of complex I and supercomplex I + III. Further studies of complex I and its supercomplexes in diverse organisms are needed to determine the universal and clade-specific mechanisms of respiration. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_29203.map.gz emd_29203.map.gz | 777.7 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-29203-v30.xml emd-29203-v30.xml emd-29203.xml emd-29203.xml | 21.1 KB 21.1 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_29203_fsc.xml emd_29203_fsc.xml | 20.1 KB | Display |  FSC data file FSC data file |
| Images |  emd_29203.png emd_29203.png | 58 KB | ||
| Masks |  emd_29203_msk_1.map emd_29203_msk_1.map | 824 MB |  Mask map Mask map | |
| Others |  emd_29203_half_map_1.map.gz emd_29203_half_map_1.map.gz emd_29203_half_map_2.map.gz emd_29203_half_map_2.map.gz | 765.7 MB 765.7 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-29203 http://ftp.pdbj.org/pub/emdb/structures/EMD-29203 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-29203 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-29203 | HTTPS FTP |
-Validation report
| Summary document |  emd_29203_validation.pdf.gz emd_29203_validation.pdf.gz | 969.8 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_29203_full_validation.pdf.gz emd_29203_full_validation.pdf.gz | 969.4 KB | Display | |
| Data in XML |  emd_29203_validation.xml.gz emd_29203_validation.xml.gz | 29.3 KB | Display | |
| Data in CIF |  emd_29203_validation.cif.gz emd_29203_validation.cif.gz | 38.6 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-29203 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-29203 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-29203 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-29203 | HTTPS FTP |
-Related structure data
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_29203.map.gz / Format: CCP4 / Size: 824 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_29203.map.gz / Format: CCP4 / Size: 824 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Bridgeless supercomplex I III2 class 4_mask | ||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.88 Å | ||||||||||||||||||||||||||||||||||||
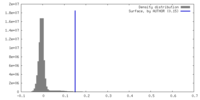
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Mask #1
| File |  emd_29203_msk_1.map emd_29203_msk_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
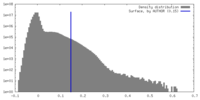
| Density Histograms |
-Half map: Bridgeless supercomplex I III2 class 4 half map A
| File | emd_29203_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Bridgeless supercomplex I III2 class 4_half map A | ||||||||||||
| Projections & Slices |
| ||||||||||||
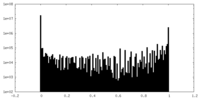
| Density Histograms |
-Half map: Bridgeless supercomplex I III2 class 4 half map B
| File | emd_29203_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Bridgeless supercomplex I III2 class 4_half map B | ||||||||||||
| Projections & Slices |
| ||||||||||||
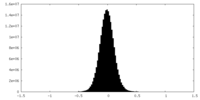
| Density Histograms |
- Sample components
Sample components
-Entire : Vigna radiata supercomplex I+III2 (full bridge)
| Entire | Name: Vigna radiata supercomplex I+III2 (full bridge) |
|---|---|
| Components |
|
-Supramolecule #1: Vigna radiata supercomplex I+III2 (full bridge)
| Supramolecule | Name: Vigna radiata supercomplex I+III2 (full bridge) / type: complex / ID: 1 / Chimera: Yes / Parent: 0 / Macromolecule list: #1-#59 Details: Higher-order assembly between respiratory complex I and complex III2 |
|---|---|
| Source (natural) | Organism:  Vigna radiata (mung bean) Vigna radiata (mung bean) |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 1.55 mg/mL |
|---|---|
| Buffer | pH: 7.7 Details: 0.2% digitonin, 30 mM HEPES pH 7.7, 150 mM potassium acetate, 1 mM EDTA, 0.002% PMSF |
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 90 % / Instrument: LEICA EM GP / Details: 4 ul, 20 seconds pre-blot, blot 4 seconds. |
| Details | Digitonin-extracted, amphipol (A8-35)stabilized |
- Electron microscopy
Electron microscopy
| Microscope | TFS GLACIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Number grids imaged: 2 / Number real images: 25712 / Average exposure time: 3.0 sec. / Average electron dose: 60.0 e/Å2 |
| Electron beam | Acceleration voltage: 200 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: OTHER / Imaging mode: OTHER / Nominal defocus max: 2.5 µm / Nominal defocus min: 0.5 µm |
| Sample stage | Cooling holder cryogen: NITROGEN |
- Image processing
Image processing
-Atomic model buiding 1
| Refinement | Space: REAL / Protocol: RIGID BODY FIT |
|---|
 Movie
Movie Controller
Controller


















 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)