+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | CryoEM structure of designed modular protein oligomer C4-131 | |||||||||
 Map data Map data | designed modular protein oligomer C4-131 | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | Synthetic / Self-assembling / Oligomeric / Helical repeats / DE NOVO PROTEIN | |||||||||
| Biological species | synthetic construct (others) | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 9.1 Å | |||||||||
 Authors Authors | Redler RL / Edman NI / Baker D / Ekiert D / Bhabha G | |||||||||
| Funding support |  United States, 2 items United States, 2 items
| |||||||||
 Citation Citation | Journal: bioRxiv / Year: 2023 Title: Modulation of FGF pathway signaling and vascular differentiation using designed oligomeric assemblies. Authors: Natasha I Edman / Rachel L Redler / Ashish Phal / Thomas Schlichthaerle / Sanjay R Srivatsan / Ali Etemadi / Seong J An / Andrew Favor / Devon Ehnes / Zhe Li / Florian Praetorius / Max ...Authors: Natasha I Edman / Rachel L Redler / Ashish Phal / Thomas Schlichthaerle / Sanjay R Srivatsan / Ali Etemadi / Seong J An / Andrew Favor / Devon Ehnes / Zhe Li / Florian Praetorius / Max Gordon / Wei Yang / Brian Coventry / Derrick R Hicks / Longxing Cao / Neville Bethel / Piper Heine / Analisa Murray / Stacey Gerben / Lauren Carter / Marcos Miranda / Babak Negahdari / Sangwon Lee / Cole Trapnell / Lance Stewart / Damian C Ekiert / Joseph Schlessinger / Jay Shendure / Gira Bhabha / Hannele Ruohola-Baker / David Baker /   Abstract: Growth factors and cytokines signal by binding to the extracellular domains of their receptors and drive association and transphosphorylation of the receptor intracellular tyrosine kinase domains, ...Growth factors and cytokines signal by binding to the extracellular domains of their receptors and drive association and transphosphorylation of the receptor intracellular tyrosine kinase domains, initiating downstream signaling cascades. To enable systematic exploration of how receptor valency and geometry affects signaling outcomes, we designed cyclic homo-oligomers with up to 8 subunits using repeat protein building blocks that can be modularly extended. By incorporating a designed fibroblast growth-factor receptor (FGFR) binding module into these scaffolds, we generated a series of synthetic signaling ligands that exhibit potent valency- and geometry-dependent Ca2+ release and MAPK pathway activation. The high specificity of the designed agonists reveal distinct roles for two FGFR splice variants in driving endothelial and mesenchymal cell fates during early vascular development. The ability to incorporate receptor binding domains and repeat extensions in a modular fashion makes our designed scaffolds broadly useful for probing and manipulating cellular signaling pathways. #2:  Journal: Cell(Cambridge,Mass.) / Year: 2024 Journal: Cell(Cambridge,Mass.) / Year: 2024Title: Modulation of FGF pathway signaling and vascular differentiation using designed oligomeric assemblies Authors: Edman NI / Redler RL / Phal A / Schlichthaerle T / Srivatsan SR / Etemadi A / An SJ / Favor A / Ehnes D / Li Z / Praetorius F / Gordon M / Yang W / Coventry B / Hicks DR / Cao L / Bethel N / ...Authors: Edman NI / Redler RL / Phal A / Schlichthaerle T / Srivatsan SR / Etemadi A / An SJ / Favor A / Ehnes D / Li Z / Praetorius F / Gordon M / Yang W / Coventry B / Hicks DR / Cao L / Bethel N / Heine P / Murray A / Gerben S / Carter L / Miranda M / Negahdari B / Lee S / Trapnell C / Stewart L / Ekiert DC / Schlessinger J / Shendure J / Bhabha G / Ruohola-Baker H / Baker D | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_28958.map.gz emd_28958.map.gz | 6.4 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-28958-v30.xml emd-28958-v30.xml emd-28958.xml emd-28958.xml | 22.7 KB 22.7 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_28958.png emd_28958.png | 64.1 KB | ||
| Filedesc metadata |  emd-28958.cif.gz emd-28958.cif.gz | 5.9 KB | ||
| Others |  emd_28958_half_map_1.map.gz emd_28958_half_map_1.map.gz emd_28958_half_map_2.map.gz emd_28958_half_map_2.map.gz | 11.9 MB 11.9 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-28958 http://ftp.pdbj.org/pub/emdb/structures/EMD-28958 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-28958 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-28958 | HTTPS FTP |
-Related structure data
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_28958.map.gz / Format: CCP4 / Size: 12.9 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_28958.map.gz / Format: CCP4 / Size: 12.9 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | designed modular protein oligomer C4-131 | ||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.096 Å | ||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Half map: Half Map 1
| File | emd_28958_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Half Map 1 | ||||||||||||
| Projections & Slices |
| ||||||||||||
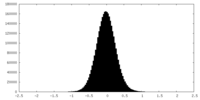
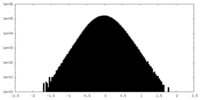
| Density Histograms |
-Half map: Half Map 2
| File | emd_28958_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Half Map 2 | ||||||||||||
| Projections & Slices |
| ||||||||||||
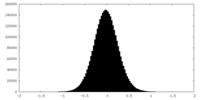
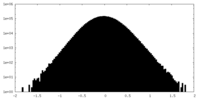
| Density Histograms |
- Sample components
Sample components
-Entire : Self-assembled homo-tetramer of de novo designed protein C4-131
| Entire | Name: Self-assembled homo-tetramer of de novo designed protein C4-131 |
|---|---|
| Components |
|
-Supramolecule #1: Self-assembled homo-tetramer of de novo designed protein C4-131
| Supramolecule | Name: Self-assembled homo-tetramer of de novo designed protein C4-131 type: complex / ID: 1 / Parent: 0 / Macromolecule list: all |
|---|---|
| Source (natural) | Organism: synthetic construct (others) |
-Macromolecule #1: C4-131
| Macromolecule | Name: C4-131 / type: protein_or_peptide / ID: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism: synthetic construct (others) |
| Recombinant expression | Organism:  |
| Sequence | String: MGLKELLKRA EELAKSPDPE DLKEAVRLAE EVVRERPGSE AAKKALEIIQ EAAEKLKKSP DPEAIIAAAR ALLKIAATTG DNEAAKQAIE AASKAAQLAE QRGDDELVCE ALALLIAAQV LLLKQQGVPM LEVAIHVAET ILQILQRLKR KGASEEVRKE CLKRILREIA ...String: MGLKELLKRA EELAKSPDPE DLKEAVRLAE EVVRERPGSE AAKKALEIIQ EAAEKLKKSP DPEAIIAAAR ALLKIAATTG DNEAAKQAIE AASKAAQLAE QRGDDELVCE ALALLIAAQV LLLKQQGVPM LEVAIHVAET ILQILQRLKR KGASEEVRKE CLKRILREIA EALQRSGVPE EEIALIMLLI ILLLMMLGSL EHHHHHH |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 0.75 mg/mL |
|---|---|
| Buffer | pH: 8 |
| Grid | Model: Quantifoil R2/2 / Material: COPPER / Mesh: 300 / Support film - #0 - Film type ID: 1 / Support film - #0 - Material: CARBON / Support film - #0 - topology: HOLEY ARRAY / Support film - #1 - Film type ID: 2 / Support film - #1 - Material: CARBON / Support film - #1 - topology: CONTINUOUS / Support film - #1 - Film thickness: 2 / Pretreatment - Type: GLOW DISCHARGE / Pretreatment - Time: 5 sec. |
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 295 K / Instrument: FEI VITROBOT MARK IV / Details: blot time = 4s; blot force = 0. |
- Electron microscopy
Electron microscopy
| Microscope | FEI TALOS ARCTICA |
|---|---|
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Number grids imaged: 1 / Number real images: 26 / Average exposure time: 2.8 sec. / Average electron dose: 56.91 e/Å2 |
| Electron beam | Acceleration voltage: 200 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: 2.9 µm / Nominal defocus min: 2.1 µm / Nominal magnification: 36000 |
| Experimental equipment |  Model: Talos Arctica / Image courtesy: FEI Company |
- Image processing
Image processing
| Startup model | Type of model: NONE / Details: Ab initio model generated in Cryosparc |
|---|---|
| Final reconstruction | Applied symmetry - Point group: C1 (asymmetric) / Resolution.type: BY AUTHOR / Resolution: 9.1 Å / Resolution method: FSC 0.143 CUT-OFF / Software - Name: cryoSPARC (ver. 3) / Number images used: 13986 |
| Initial angle assignment | Type: MAXIMUM LIKELIHOOD / Software - Name: cryoSPARC (ver. 3) |
| Final angle assignment | Type: MAXIMUM LIKELIHOOD / Software - Name: cryoSPARC (ver. 3) |
 Movie
Movie Controller
Controller

















 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)