[English] 日本語
 Yorodumi
Yorodumi- EMDB-28943: TMEM106B doublet filaments extracted from MSTD neurodegenerative ... -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | TMEM106B doublet filaments extracted from MSTD neurodegenerative human brain | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | TMEM106B / TMEM filament / MSTD / NEUROPEPTIDE | |||||||||
| Function / homology |  Function and homology information Function and homology informationlysosomal protein catabolic process / lysosomal lumen acidification / regulation of lysosome organization / lysosome localization / positive regulation of dendrite development / lysosomal transport / lysosome organization / dendrite morphogenesis / neuron cellular homeostasis / late endosome membrane ...lysosomal protein catabolic process / lysosomal lumen acidification / regulation of lysosome organization / lysosome localization / positive regulation of dendrite development / lysosomal transport / lysosome organization / dendrite morphogenesis / neuron cellular homeostasis / late endosome membrane / ATPase binding / lysosome / endosome / lysosomal membrane / plasma membrane Similarity search - Function | |||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | |||||||||
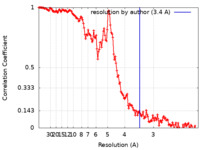
| Method | helical reconstruction / cryo EM / Resolution: 3.4 Å | |||||||||
 Authors Authors | Hoq MR / Bharath SR / Jiang W | |||||||||
| Funding support |  United States, 1 items United States, 1 items
| |||||||||
 Citation Citation |  Journal: Acta Neuropathol / Year: 2023 Journal: Acta Neuropathol / Year: 2023Title: Cross-β helical filaments of Tau and TMEM106B in gray and white matter of multiple system tauopathy with presenile dementia. Authors: Md Rejaul Hoq / Sakshibeedu R Bharath / Grace I Hallinan / Anllely Fernandez / Frank S Vago / Kadir A Ozcan / Daoyi Li / Holly J Garringer / Ruben Vidal / Bernardino Ghetti / Wen Jiang /  | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_28943.map.gz emd_28943.map.gz | 78.5 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-28943-v30.xml emd-28943-v30.xml emd-28943.xml emd-28943.xml | 14.2 KB 14.2 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_28943_fsc.xml emd_28943_fsc.xml | 18.1 KB | Display |  FSC data file FSC data file |
| Images |  emd_28943.png emd_28943.png | 79.9 KB | ||
| Filedesc metadata |  emd-28943.cif.gz emd-28943.cif.gz | 5.5 KB | ||
| Others |  emd_28943_half_map_1.map.gz emd_28943_half_map_1.map.gz emd_28943_half_map_2.map.gz emd_28943_half_map_2.map.gz | 67 MB 67 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-28943 http://ftp.pdbj.org/pub/emdb/structures/EMD-28943 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-28943 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-28943 | HTTPS FTP |
-Related structure data
| Related structure data |  8f9kMC  7tmcC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_28943.map.gz / Format: CCP4 / Size: 244.1 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_28943.map.gz / Format: CCP4 / Size: 244.1 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.06 Å | ||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Half map: #2
| File | emd_28943_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: #1
| File | emd_28943_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : TMEM106B
| Entire | Name: TMEM106B |
|---|---|
| Components |
|
-Supramolecule #1: TMEM106B
| Supramolecule | Name: TMEM106B / type: tissue / ID: 1 / Parent: 0 / Macromolecule list: #1 |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
-Macromolecule #1: Transmembrane protein 106B
| Macromolecule | Name: Transmembrane protein 106B / type: protein_or_peptide / ID: 1 / Number of copies: 6 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 31.156318 KDa |
| Recombinant expression | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: MGKSLSHLPL HSSKEDAYDG VTSENMRNGL VNSEVHNEDG RNGDVSQFPY VEFTGRDSVT CPTCQGTGRI PRGQENQLVA LIPYSDQRL RPRRTKLYVM ASVFVCLLLS GLAVFFLFPR SIDVKYIGVK SAYVSYDVQK RTIYLNITNT LNITNNNYYS V EVENITAQ ...String: MGKSLSHLPL HSSKEDAYDG VTSENMRNGL VNSEVHNEDG RNGDVSQFPY VEFTGRDSVT CPTCQGTGRI PRGQENQLVA LIPYSDQRL RPRRTKLYVM ASVFVCLLLS GLAVFFLFPR SIDVKYIGVK SAYVSYDVQK RTIYLNITNT LNITNNNYYS V EVENITAQ VQFSKTVIGK ARLNNITIIG PLDMKQIDYT VPTVIAEEMS YMYDFCTLIS IKVHNIVLMM QVTVTTTYFG HS EQISQER YQYVDCGRNT TYQLGQSEYL NVLQPQQ UniProtKB: Transmembrane protein 106B |
-Macromolecule #2: 2-acetamido-2-deoxy-beta-D-glucopyranose
| Macromolecule | Name: 2-acetamido-2-deoxy-beta-D-glucopyranose / type: ligand / ID: 2 / Number of copies: 24 / Formula: NAG |
|---|---|
| Molecular weight | Theoretical: 221.208 Da |
| Chemical component information |  ChemComp-NAG: |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | helical reconstruction |
| Aggregation state | filament |
- Sample preparation
Sample preparation
| Concentration | 1 mg/mL |
|---|---|
| Buffer | pH: 7.2 |
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | TFS KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Average exposure time: 1.103 sec. / Average electron dose: 50.46 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | C2 aperture diameter: 100.0 µm / Illumination mode: SPOT SCAN / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm / Nominal defocus max: 5.0 µm / Nominal defocus min: 0.5 µm / Nominal magnification: 81000 |
| Sample stage | Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller



 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)