+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-2874 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo electron microscopy of SNAP-SNARE assembly in 20S particle | |||||||||
 Map data Map data | Reconstruction of alpha-SNAP-SNARE assembly in 20S particle | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | 20S particles / SNARE / alpha-SNAP / membrane fusion | |||||||||
| Biological species |   | |||||||||
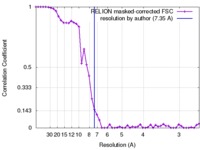
| Method | single particle reconstruction / cryo EM / Resolution: 7.35 Å | |||||||||
 Authors Authors | Zhou Q / Huang X / Sun S / Li XM / Wang HW / Sui SF | |||||||||
 Citation Citation |  Journal: Cell Res / Year: 2015 Journal: Cell Res / Year: 2015Title: Cryo-EM structure of SNAP-SNARE assembly in 20S particle. Authors: Qiang Zhou / Xuan Huang / Shan Sun / Xueming Li / Hong-Wei Wang / Sen-Fang Sui /  Abstract: N-ethylmaleimide-sensitive factor (NSF) and α soluble NSF attachment proteins (α-SNAPs) work together within a 20S particle to disassemble and recycle the SNAP receptor (SNARE) complex after ...N-ethylmaleimide-sensitive factor (NSF) and α soluble NSF attachment proteins (α-SNAPs) work together within a 20S particle to disassemble and recycle the SNAP receptor (SNARE) complex after intracellular membrane fusion. To understand the disassembly mechanism of the SNARE complex by NSF and α-SNAP, we performed single-particle cryo-electron microscopy analysis of 20S particles and determined the structure of the α-SNAP-SNARE assembly portion at a resolution of 7.35 Å. The structure illustrates that four α-SNAPs wrap around the single left-handed SNARE helical bundle as a right-handed cylindrical assembly within a 20S particle. A conserved hydrophobic patch connecting helices 9 and 10 of each α-SNAP forms a chock protruding into the groove of the SNARE four-helix bundle. Biochemical studies proved that this structural element was critical for SNARE complex disassembly. Our study suggests how four α-SNAPs may coordinate with the NSF to tear the SNARE complex into individual proteins. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_2874.map.gz emd_2874.map.gz | 7.2 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-2874-v30.xml emd-2874-v30.xml emd-2874.xml emd-2874.xml | 12.4 KB 12.4 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_2874_fsc.xml emd_2874_fsc.xml | 4.5 KB | Display |  FSC data file FSC data file |
| Images |  emd_2874.png emd_2874.png | 89.9 KB | ||
| Masks |  emd_2874_msk_1.map emd_2874_msk_1.map | 8 MB |  Mask map Mask map | |
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-2874 http://ftp.pdbj.org/pub/emdb/structures/EMD-2874 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-2874 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-2874 | HTTPS FTP |
-Validation report
| Summary document |  emd_2874_validation.pdf.gz emd_2874_validation.pdf.gz | 274 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_2874_full_validation.pdf.gz emd_2874_full_validation.pdf.gz | 273.1 KB | Display | |
| Data in XML |  emd_2874_validation.xml.gz emd_2874_validation.xml.gz | 7.8 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-2874 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-2874 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-2874 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-2874 | HTTPS FTP |
-Related structure data
| Similar structure data |
|---|
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_2874.map.gz / Format: CCP4 / Size: 7.8 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_2874.map.gz / Format: CCP4 / Size: 7.8 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Reconstruction of alpha-SNAP-SNARE assembly in 20S particle | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.32 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
-Segmentation: This is a soft spherical mask.
| Annotation | This is a soft spherical mask. | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| File |  emd_2874_msk_1.map emd_2874_msk_1.map | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : alpha-SNAP-SNARE assembly in 20S particle
| Entire | Name: alpha-SNAP-SNARE assembly in 20S particle |
|---|---|
| Components |
|
-Supramolecule #1000: alpha-SNAP-SNARE assembly in 20S particle
| Supramolecule | Name: alpha-SNAP-SNARE assembly in 20S particle / type: sample / ID: 1000 / Details: 20S particle was prepared in nanodisc. Oligomeric state: One homotetramer of alpha-SNAP binds to one monomer of SNARE complex. Number unique components: 2 |
|---|---|
| Molecular weight | Theoretical: 200 KDa |
-Macromolecule #1: soluble NSF attachment protein receptor
| Macromolecule | Name: soluble NSF attachment protein receptor / type: protein_or_peptide / ID: 1 / Name.synonym: SNARE / Number of copies: 1 / Oligomeric state: monomer / Recombinant expression: Yes |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 66 KDa |
| Recombinant expression | Organism:  |
-Macromolecule #2: alpha soluble NSF attachment protein
| Macromolecule | Name: alpha soluble NSF attachment protein / type: protein_or_peptide / ID: 2 / Name.synonym: alpha-SNAP / Number of copies: 4 / Oligomeric state: tetramer / Recombinant expression: Yes |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 33 KDa |
| Recombinant expression | Organism:  |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 0.2 mg/mL |
|---|---|
| Grid | Details: 400 mesh copper grid with thin carbon support |
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Instrument: FEI VITROBOT MARK IV / Method: Blot for 1 second before plunging |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Alignment procedure | Legacy - Astigmatism: Objective lens astigmatism was corrected at 22,500 times magnification. |
| Date | Jul 2, 2014 |
| Image recording | Category: CCD / Film or detector model: GATAN K2 (4k x 4k) / Number real images: 2349 / Average electron dose: 46 e/Å2 Details: Every image is the average of motion-corrected movie frames. |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm / Nominal defocus max: 2.5 µm / Nominal defocus min: 1.5 µm / Nominal magnification: 22500 |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
- Image processing
Image processing
-Atomic model buiding 1
| Initial model | PDB ID: Chain - #0 - Chain ID: A / Chain - #1 - Chain ID: B / Chain - #2 - Chain ID: C / Chain - #3 - Chain ID: D |
|---|---|
| Software | Name:  Chimera Chimera |
| Refinement | Space: REAL / Protocol: RIGID BODY FIT |
 Movie
Movie Controller
Controller












 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)