[English] 日本語
 Yorodumi
Yorodumi- EMDB-28224: CryoEM structure of Resistance to Inhibitors of Cholinesterase-8B... -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
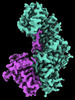
| Title | CryoEM structure of Resistance to Inhibitors of Cholinesterase-8B (Ric-8B) in complex with olfactory G protein alpha olf | |||||||||
 Map data Map data | Sharpened map | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | chaperone / armadillo repeat / GEF / Ras / SIGNALING PROTEIN | |||||||||
| Function / homology |  Function and homology information Function and homology informationAdenylate cyclase activating pathway / sensory perception of chemical stimulus / response to caffeine / G-protein alpha-subunit binding / Adenylate cyclase inhibitory pathway / adenylate cyclase regulator activity / protein folding chaperone / guanyl-nucleotide exchange factor activity / response to amphetamine / G protein-coupled receptor binding ...Adenylate cyclase activating pathway / sensory perception of chemical stimulus / response to caffeine / G-protein alpha-subunit binding / Adenylate cyclase inhibitory pathway / adenylate cyclase regulator activity / protein folding chaperone / guanyl-nucleotide exchange factor activity / response to amphetamine / G protein-coupled receptor binding / G-protein beta/gamma-subunit complex binding / Olfactory Signaling Pathway / adenylate cyclase-activating G protein-coupled receptor signaling pathway / sensory perception of smell / adenylate cyclase-activating dopamine receptor signaling pathway / heterotrimeric G-protein complex / G protein activity / cell cortex / Hydrolases; Acting on acid anhydrides; Acting on GTP to facilitate cellular and subcellular movement / G protein-coupled receptor signaling pathway / GTPase activity / centrosome / GTP binding / signal transduction / extracellular exosome / metal ion binding / plasma membrane / cytoplasm / cytosol Similarity search - Function | |||||||||
| Biological species |  Homo sapiens (human) / Homo sapiens (human) /  | |||||||||
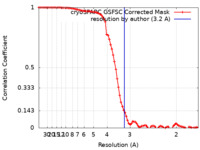
| Method | single particle reconstruction / cryo EM / Resolution: 3.2 Å | |||||||||
 Authors Authors | Papasergi-Scott MM / Skiniotis G | |||||||||
| Funding support |  United States, 1 items United States, 1 items
| |||||||||
 Citation Citation |  Journal: Structure / Year: 2023 Journal: Structure / Year: 2023Title: Structures of Ric-8B in complex with Gα protein folding clients reveal isoform specificity mechanisms. Authors: Makaía M Papasergi-Scott / Frank E Kwarcinski / Maiya Yu / Ouliana Panova / Ann M Ovrutsky / Georgios Skiniotis / Gregory G Tall /  Abstract: Mammalian Ric-8 proteins act as chaperones to regulate the cellular abundance of heterotrimeric G protein α subunits. The Ric-8A isoform chaperones Gαi/o, Gα12/13, and Gαq/11 subunits, while Ric- ...Mammalian Ric-8 proteins act as chaperones to regulate the cellular abundance of heterotrimeric G protein α subunits. The Ric-8A isoform chaperones Gαi/o, Gα12/13, and Gαq/11 subunits, while Ric-8B acts on Gαs/olf subunits. Here, we determined cryoelectron microscopy (cryo-EM) structures of Ric-8B in complex with Gαs and Gαolf, revealing isoform differences in the relative positioning and contacts between the C-terminal α5 helix of Gα within the concave pocket formed by Ric-8 α-helical repeat elements. Despite the overall architectural similarity with our earlier structures of Ric-8A complexed to Gαq and Gαi1, Ric-8B distinctly accommodates an extended loop found only in Gαs/olf proteins. The structures, along with results from Ric-8 protein thermal stability assays and cell-based Gαolf folding assays, support a requirement for the Gα C-terminal region for binding specificity, and highlight that multiple structural elements impart specificity for Ric-8/G protein binding. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_28224.map.gz emd_28224.map.gz | 54.7 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-28224-v30.xml emd-28224-v30.xml emd-28224.xml emd-28224.xml | 17.7 KB 17.7 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_28224_fsc.xml emd_28224_fsc.xml | 9.3 KB | Display |  FSC data file FSC data file |
| Images |  emd_28224.png emd_28224.png | 125.8 KB | ||
| Masks |  emd_28224_msk_1.map emd_28224_msk_1.map | 58.2 MB |  Mask map Mask map | |
| Filedesc metadata |  emd-28224.cif.gz emd-28224.cif.gz | 6.3 KB | ||
| Others |  emd_28224_half_map_1.map.gz emd_28224_half_map_1.map.gz emd_28224_half_map_2.map.gz emd_28224_half_map_2.map.gz | 53.9 MB 53.9 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-28224 http://ftp.pdbj.org/pub/emdb/structures/EMD-28224 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-28224 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-28224 | HTTPS FTP |
-Validation report
| Summary document |  emd_28224_validation.pdf.gz emd_28224_validation.pdf.gz | 954.4 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_28224_full_validation.pdf.gz emd_28224_full_validation.pdf.gz | 954 KB | Display | |
| Data in XML |  emd_28224_validation.xml.gz emd_28224_validation.xml.gz | 16.3 KB | Display | |
| Data in CIF |  emd_28224_validation.cif.gz emd_28224_validation.cif.gz | 20.9 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-28224 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-28224 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-28224 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-28224 | HTTPS FTP |
-Related structure data
| Related structure data |  8el8MC  8el7C M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_28224.map.gz / Format: CCP4 / Size: 58.2 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_28224.map.gz / Format: CCP4 / Size: 58.2 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Sharpened map | ||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.8521 Å | ||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Mask #1
| File |  emd_28224_msk_1.map emd_28224_msk_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
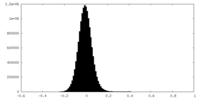
| Density Histograms |
-Half map: Half map 2
| File | emd_28224_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Half map 2 | ||||||||||||
| Projections & Slices |
| ||||||||||||
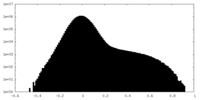
| Density Histograms |
-Half map: Half map 1
| File | emd_28224_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Half map 1 | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : Ric-8B in complex with G protein subunit alpha olf
| Entire | Name: Ric-8B in complex with G protein subunit alpha olf |
|---|---|
| Components |
|
-Supramolecule #1: Ric-8B in complex with G protein subunit alpha olf
| Supramolecule | Name: Ric-8B in complex with G protein subunit alpha olf / type: complex / ID: 1 / Parent: 0 / Macromolecule list: all |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
-Macromolecule #1: Guanine nucleotide-binding protein G(olf) subunit alpha
| Macromolecule | Name: Guanine nucleotide-binding protein G(olf) subunit alpha type: protein_or_peptide / ID: 1 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 44.37043 KDa |
| Recombinant expression | Organism:  Trichoplusia ni (cabbage looper) Trichoplusia ni (cabbage looper) |
| Sequence | String: MGCLGGNSKT TEDQGVDEKE RREANKKIEK QLQKERLAYK ATHRLLLLGA GESGKSTIVK QMRILHVNGF NPEEKKQKIL DIRKNVKDA IVTIVSAMST IIPPVPLANP ENQFRSDYIK SIAPITDFEY SQEFFDHVKK LWDDEGVKAC FERSNEYQLI D CAQYFLER ...String: MGCLGGNSKT TEDQGVDEKE RREANKKIEK QLQKERLAYK ATHRLLLLGA GESGKSTIVK QMRILHVNGF NPEEKKQKIL DIRKNVKDA IVTIVSAMST IIPPVPLANP ENQFRSDYIK SIAPITDFEY SQEFFDHVKK LWDDEGVKAC FERSNEYQLI D CAQYFLER IDSVSLVDYT PTDQDLLRCR VLTSGIFETR FQVDKVNFHM FDVGGQRDER RKWIQCFNDV TAIIYVAACS SY NMVIRED NNTNRLRESL DLFESIWNNR WLRTISIILF LNKQDMLAEK VLAGKSKIED YFPEYANYTV PEDATPDAGE DPK VTRAKF FIRDLFLRIS TATGDGKHYC YPHFTCAVDT ENIRRVFNDC RDIIQRMHLK QYELL UniProtKB: Guanine nucleotide-binding protein G(olf) subunit alpha |
-Macromolecule #2: Isoform 1 of Synembryn-B
| Macromolecule | Name: Isoform 1 of Synembryn-B / type: protein_or_peptide / ID: 2 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 63.928043 KDa |
| Recombinant expression | Organism:  Trichoplusia ni (cabbage looper) Trichoplusia ni (cabbage looper) |
| Sequence | String: GEFMDEERAL YIVRAGEAGA IERVLRDYSD KHRATFKFES ADEDKRKKLC EGIFKVLVKE VPTTCQVSCL EVLRILSRDK KILVPVTTK ENMQILLRLA KLHESDDSLE KVSEFPVIVE SLKCLCNIVF NSQMAQQLSL ELNLAAKLCN LLRKCKDRKF I NDIKCFDL ...String: GEFMDEERAL YIVRAGEAGA IERVLRDYSD KHRATFKFES ADEDKRKKLC EGIFKVLVKE VPTTCQVSCL EVLRILSRDK KILVPVTTK ENMQILLRLA KLHESDDSLE KVSEFPVIVE SLKCLCNIVF NSQMAQQLSL ELNLAAKLCN LLRKCKDRKF I NDIKCFDL RLLFVLSLLH TDIRSQLRYE LQGLPLLTQI LESAFSIKWT DEYESAIDHN GPPLSPQETD CAIEALKALF NV TVDSWKV HKESDSHQFR VMAAVLRHCL LIVGPTEDKT EELHSNAVNL LSNVPVSCLD VLICPLTHEE TAQEAATLDE LPS DKTTEK DTALKNSTMV YNGMNMEAIH VLLNFMEKRI DKGSSYREGL TPVLSLLTEC SRAHRNIRKF LKDQVLPPLR DVTN RPEVG STVRNKLVRL MTHVDLGVKQ IAAEFLFVLC KERVDSLLKY TGYGNAAGLL AARGLLAGGR GDNWY(SEP)EDED (TPO)DTEEYKNA KPNINLITGH LEEPMPNPID EMTEEQKEYE AMKLVNMLDK LSREELLKPM GLKPDGTITP LEEALSQ YS VIEETSSDTD UniProtKB: Chaperone Ric-8B |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 3.5 mg/mL |
|---|---|
| Buffer | pH: 8 |
| Grid | Model: UltrAuFoil / Material: GOLD / Pretreatment - Type: GLOW DISCHARGE |
| Vitrification | Cryogen name: ETHANE / Instrument: FEI VITROBOT MARK IV |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Number real images: 4670 / Average exposure time: 2.49 sec. / Average electron dose: 68.6 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Calibrated magnification: 57050 / Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: 1.8 µm / Nominal defocus min: 0.8 µm |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
+ Image processing
Image processing
-Atomic model buiding 1
| Initial model | PDB ID: Chain - Source name: PDB / Chain - Initial model type: experimental model |
|---|---|
| Refinement | Space: REAL / Protocol: AB INITIO MODEL / Overall B value: 171.1 |
| Output model |  PDB-8el8: |
 Movie
Movie Controller
Controller








 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)