+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo-EM structure of L9 Fab in complex with rsCSP | |||||||||
 Map data Map data | Cryo-EM structure of L9 Fab in complex with rsCSP | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | PfCSP / malaria / antibody / IMMUNE SYSTEM | |||||||||
| Function / homology |  Function and homology information Function and homology information | |||||||||
| Biological species |   Homo sapiens (human) Homo sapiens (human) | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 3.36 Å | |||||||||
 Authors Authors | Martin GM / Ward AB | |||||||||
| Funding support |  United States, 1 items United States, 1 items
| |||||||||
 Citation Citation |  Journal: Nat Commun / Year: 2023 Journal: Nat Commun / Year: 2023Title: Structural basis of epitope selectivity and potent protection from malaria by PfCSP antibody L9. Authors: Gregory M Martin / Monica L Fernández-Quintero / Wen-Hsin Lee / Tossapol Pholcharee / Lisa Eshun-Wilson / Klaus R Liedl / Marie Pancera / Robert A Seder / Ian A Wilson / Andrew B Ward /    Abstract: A primary objective in malaria vaccine design is the generation of high-quality antibody responses against the circumsporozoite protein of the malaria parasite, Plasmodium falciparum (PfCSP). To ...A primary objective in malaria vaccine design is the generation of high-quality antibody responses against the circumsporozoite protein of the malaria parasite, Plasmodium falciparum (PfCSP). To enable rational antigen design, we solved a cryo-EM structure of the highly potent anti-PfCSP antibody L9 in complex with recombinant PfCSP. We found that L9 Fab binds multivalently to the minor (NPNV) repeat domain, which is stabilized by a unique set of affinity-matured homotypic, antibody-antibody contacts. Molecular dynamics simulations revealed a critical role of the L9 light chain in integrity of the homotypic interface, which likely impacts PfCSP affinity and protective efficacy. These findings reveal the molecular mechanism of the unique NPNV selectivity of L9 and emphasize the importance of anti-homotypic affinity maturation in protective immunity against P. falciparum. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_28135.map.gz emd_28135.map.gz | 20.7 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-28135-v30.xml emd-28135-v30.xml emd-28135.xml emd-28135.xml | 17.1 KB 17.1 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_28135.png emd_28135.png | 112.7 KB | ||
| Filedesc metadata |  emd-28135.cif.gz emd-28135.cif.gz | 5.7 KB | ||
| Others |  emd_28135_half_map_1.map.gz emd_28135_half_map_1.map.gz emd_28135_half_map_2.map.gz emd_28135_half_map_2.map.gz | 20.7 MB 20.7 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-28135 http://ftp.pdbj.org/pub/emdb/structures/EMD-28135 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-28135 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-28135 | HTTPS FTP |
-Validation report
| Summary document |  emd_28135_validation.pdf.gz emd_28135_validation.pdf.gz | 792.3 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_28135_full_validation.pdf.gz emd_28135_full_validation.pdf.gz | 791.9 KB | Display | |
| Data in XML |  emd_28135_validation.xml.gz emd_28135_validation.xml.gz | 10.2 KB | Display | |
| Data in CIF |  emd_28135_validation.cif.gz emd_28135_validation.cif.gz | 11.9 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-28135 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-28135 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-28135 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-28135 | HTTPS FTP |
-Related structure data
| Related structure data |  8eh5MC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_28135.map.gz / Format: CCP4 / Size: 22.2 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_28135.map.gz / Format: CCP4 / Size: 22.2 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Cryo-EM structure of L9 Fab in complex with rsCSP | ||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.045 Å | ||||||||||||||||||||||||||||||||||||
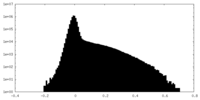
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Half map: Cryo-EM structure of L9 Fab in complex with rsCSP
| File | emd_28135_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Cryo-EM structure of L9 Fab in complex with rsCSP | ||||||||||||
| Projections & Slices |
| ||||||||||||
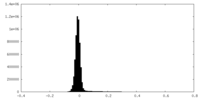
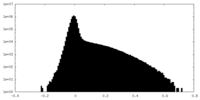
| Density Histograms |
-Half map: Cryo-EM structure of L9 Fab in complex with rsCSP
| File | emd_28135_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Cryo-EM structure of L9 Fab in complex with rsCSP | ||||||||||||
| Projections & Slices |
| ||||||||||||
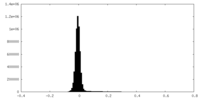
| Density Histograms |
- Sample components
Sample components
-Entire : trimeric complex of L9 Fab with rsCSP
| Entire | Name: trimeric complex of L9 Fab with rsCSP |
|---|---|
| Components |
|
-Supramolecule #1: trimeric complex of L9 Fab with rsCSP
| Supramolecule | Name: trimeric complex of L9 Fab with rsCSP / type: complex / ID: 1 / Parent: 0 / Macromolecule list: all |
|---|
-Supramolecule #2: Circumsporozoite protein
| Supramolecule | Name: Circumsporozoite protein / type: complex / ID: 2 / Parent: 1 / Macromolecule list: #1 |
|---|---|
| Source (natural) | Organism:  |
-Supramolecule #3: L9 Heavy chain, L9 Light chain
| Supramolecule | Name: L9 Heavy chain, L9 Light chain / type: complex / ID: 3 / Parent: 1 / Macromolecule list: #2-#3 |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
-Macromolecule #1: Circumsporozoite protein
| Macromolecule | Name: Circumsporozoite protein / type: protein_or_peptide / ID: 1 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 30.363354 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MYGSSSNTRV LNELNYDNAG TNLYNELEMN YYGKQENWYS LKKNSRSLGE NDDGNNEDNE KLRKPKHKKL KQPADGNPDP NANPNVDPN ANPNVDPNAN PNVDPNANPN ANPNANPNAN PNANPNANPN ANPNANPNAN PNANPNANPN ANPNANPNAN P NANPNANP ...String: MYGSSSNTRV LNELNYDNAG TNLYNELEMN YYGKQENWYS LKKNSRSLGE NDDGNNEDNE KLRKPKHKKL KQPADGNPDP NANPNVDPN ANPNVDPNAN PNVDPNANPN ANPNANPNAN PNANPNANPN ANPNANPNAN PNANPNANPN ANPNANPNAN P NANPNANP NKNNQGNGQG HNMPNDPNRN VDENANANSA VKNNNNEEPS DKHIKEYLNK IQNSLSTEWS PCSVTCGNGI QV RIKPGSA NKPKDELDYA NDIEKKICKM EKCSSVFNVV NS UniProtKB: Circumsporozoite protein |
-Macromolecule #2: L9 Heavy chain
| Macromolecule | Name: L9 Heavy chain / type: protein_or_peptide / ID: 2 / Number of copies: 3 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 24.475436 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: QVKLVESGGG VVQPGRSLRL SCEASGFIFS TYGMHWVRQA PGKGLEWVAV IWFDGSNIYY ADSVKGRFTI SRDNSKNTVF MQMDSLRAE DTAVYYCHRN FYDGSGPFDY WGQGTLVTVS SASTKGPSVF PLAPSSKSTS GGTAALGCLV KDYFPEPVTV S WNSGALTS ...String: QVKLVESGGG VVQPGRSLRL SCEASGFIFS TYGMHWVRQA PGKGLEWVAV IWFDGSNIYY ADSVKGRFTI SRDNSKNTVF MQMDSLRAE DTAVYYCHRN FYDGSGPFDY WGQGTLVTVS SASTKGPSVF PLAPSSKSTS GGTAALGCLV KDYFPEPVTV S WNSGALTS GVHTFPAVLQ SSGLYSLSSV VTVPSSSLGT QTYICNVNHK PSNTKVDKKV EPKSCDKTH |
-Macromolecule #3: L9 Light chain
| Macromolecule | Name: L9 Light chain / type: protein_or_peptide / ID: 3 / Number of copies: 3 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 23.597254 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: DIQMTQSPST LSASVGDRVT ITCRASQFIS RWLAWYQQKP GKAPKLLIYK ASSLESGVPS RFSGSGSETH FTLTISSLQP DDVATYYCQ EYTSYGRTFG QGTKVEIKRT VAAPSVFIFP PSDEQLKSGT ASVVCLLNNF YPREAKVQWK VDNALQSGNS Q ESVTEQDS ...String: DIQMTQSPST LSASVGDRVT ITCRASQFIS RWLAWYQQKP GKAPKLLIYK ASSLESGVPS RFSGSGSETH FTLTISSLQP DDVATYYCQ EYTSYGRTFG QGTKVEIKRT VAAPSVFIFP PSDEQLKSGT ASVVCLLNNF YPREAKVQWK VDNALQSGNS Q ESVTEQDS KDSTYSLSST LTLSKADYEK HKVYACEVTH QGLSSPVTKS FNRGEC |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.4 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Detector mode: COUNTING / Average electron dose: 60.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: 2.0 µm / Nominal defocus min: 0.9 µm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
- Image processing
Image processing
| Startup model | Type of model: OTHER |
|---|---|
| Final reconstruction | Resolution.type: BY AUTHOR / Resolution: 3.36 Å / Resolution method: FSC 0.143 CUT-OFF / Number images used: 451712 |
| Initial angle assignment | Type: MAXIMUM LIKELIHOOD |
| Final angle assignment | Type: PROJECTION MATCHING |
 Movie
Movie Controller
Controller




 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)