+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo-EM structure of human ABCA7 in Digitonin | |||||||||
 Map data Map data | human ABCA7 in Digitonin | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | ABC transporter / Lipid Transporter / ABC exporter / TRANSPORT PROTEIN | |||||||||
| Function / homology |  Function and homology information Function and homology informationapolipoprotein A-I receptor activity / plasma membrane raft organization / positive regulation of engulfment of apoptotic cell / negative regulation of amyloid precursor protein biosynthetic process / phospholipid transporter activity / ABC transporters in lipid homeostasis / floppase activity / phosphatidylserine floppase activity / positive regulation of phospholipid efflux / amyloid-beta clearance by cellular catabolic process ...apolipoprotein A-I receptor activity / plasma membrane raft organization / positive regulation of engulfment of apoptotic cell / negative regulation of amyloid precursor protein biosynthetic process / phospholipid transporter activity / ABC transporters in lipid homeostasis / floppase activity / phosphatidylserine floppase activity / positive regulation of phospholipid efflux / amyloid-beta clearance by cellular catabolic process / phospholipid efflux / phosphatidylcholine floppase activity / positive regulation of amyloid-beta clearance / high-density lipoprotein particle assembly / negative regulation of endocytosis / positive regulation of protein localization to cell surface / P-type phospholipid transporter / apolipoprotein A-I-mediated signaling pathway / regulation of amyloid precursor protein catabolic process / cholesterol efflux / phospholipid translocation / amyloid-beta formation / negative regulation of PERK-mediated unfolded protein response / negative regulation of amyloid-beta formation / regulation of lipid metabolic process / ATPase-coupled transmembrane transporter activity / positive regulation of cholesterol efflux / phagocytosis / glial cell projection / protein localization to nucleus / ABC-type transporter activity / phagocytic cup / negative regulation of MAPK cascade / positive regulation of phagocytosis / visual learning / memory / ruffle membrane / cell junction / early endosome membrane / positive regulation of ERK1 and ERK2 cascade / intracellular membrane-bounded organelle / Golgi membrane / cell surface / endoplasmic reticulum / Golgi apparatus / ATP hydrolysis activity / ATP binding / plasma membrane Similarity search - Function | |||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 3.9 Å | |||||||||
 Authors Authors | Alam A / Le LTM / Thompson JR | |||||||||
| Funding support |  United States, 1 items United States, 1 items
| |||||||||
 Citation Citation |  Journal: EMBO J / Year: 2023 Journal: EMBO J / Year: 2023Title: Cryo-EM structures of human ABCA7 provide insights into its phospholipid translocation mechanisms. Authors: Le Thi My Le / James Robert Thompson / Sepehr Dehghani-Ghahnaviyeh / Shashank Pant / Phuoc Xuan Dang / Jarrod Bradley French / Takahisa Kanikeyo / Emad Tajkhorshid / Amer Alam /  Abstract: Phospholipid extrusion by ABC subfamily A (ABCA) exporters is central to cellular physiology, although the specifics of the underlying substrate interactions and transport mechanisms remain poorly ...Phospholipid extrusion by ABC subfamily A (ABCA) exporters is central to cellular physiology, although the specifics of the underlying substrate interactions and transport mechanisms remain poorly resolved at the molecular level. Here we report cryo-EM structures of lipid-embedded human ABCA7 in an open state and in a nucleotide-bound, closed state at resolutions between 3.6 and 4.0 Å. The former reveals an ordered patch of bilayer lipids traversing the transmembrane domain (TMD), while the latter reveals a lipid-free, closed TMD with a small extracellular opening. These structures offer a structural framework for both substrate entry and exit from the ABCA7 TMD and highlight conserved rigid-body motions that underlie the associated conformational transitions. Combined with functional analysis and molecular dynamics (MD) simulations, our data also shed light on lipid partitioning into the ABCA7 TMD and localized membrane perturbations that underlie ABCA7 function and have broader implications for other ABCA family transporters. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_28050.map.gz emd_28050.map.gz | 200.2 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-28050-v30.xml emd-28050-v30.xml emd-28050.xml emd-28050.xml | 19.1 KB 19.1 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_28050.png emd_28050.png | 33.8 KB | ||
| Filedesc metadata |  emd-28050.cif.gz emd-28050.cif.gz | 7.4 KB | ||
| Others |  emd_28050_half_map_1.map.gz emd_28050_half_map_1.map.gz emd_28050_half_map_2.map.gz emd_28050_half_map_2.map.gz | 171.8 MB 171.7 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-28050 http://ftp.pdbj.org/pub/emdb/structures/EMD-28050 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-28050 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-28050 | HTTPS FTP |
-Related structure data
| Related structure data |  8eebMC  8edwC  8ee6C  8eopC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_28050.map.gz / Format: CCP4 / Size: 216 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_28050.map.gz / Format: CCP4 / Size: 216 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | human ABCA7 in Digitonin | ||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.889 Å | ||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Half map: Refinement half map 1
| File | emd_28050_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Refinement half map 1 | ||||||||||||
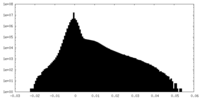
| Projections & Slices |
| ||||||||||||
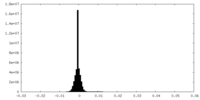
| Density Histograms |
-Half map: Refinement half map 2
| File | emd_28050_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Refinement half map 2 | ||||||||||||
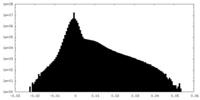
| Projections & Slices |
| ||||||||||||
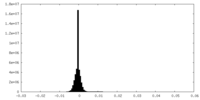
| Density Histograms |
- Sample components
Sample components
-Entire : Human ABCA7 in Digitonin
| Entire | Name: Human ABCA7 in Digitonin |
|---|---|
| Components |
|
-Supramolecule #1: Human ABCA7 in Digitonin
| Supramolecule | Name: Human ABCA7 in Digitonin / type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1 Details: Human ABCA7 recombinantly expressed in HEK293 TREX cell line and reconstituted in Digitonin |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 234.35 KDa |
-Macromolecule #1: Phospholipid-transporting ATPase ABCA7
| Macromolecule | Name: Phospholipid-transporting ATPase ABCA7 / type: protein_or_peptide / ID: 1 / Number of copies: 1 / Enantiomer: LEVO / EC number: P-type phospholipid transporter |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 234.598578 KDa |
| Recombinant expression | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: MAFWTQLMLL LWKNFMYRRR QPVQLLVELL WPLFLFFILV AVRHSHPPLE HHECHFPNKP LPSAGTVPWL QGLICNVNNT CFPQLTPGE EPGRLSNFND SLVSRLLADA RTVLGGASAH RTLAGLGKLI ATLRAARSTA QPQPTKQSPL EPPMLDVAEL L TSLLRTES ...String: MAFWTQLMLL LWKNFMYRRR QPVQLLVELL WPLFLFFILV AVRHSHPPLE HHECHFPNKP LPSAGTVPWL QGLICNVNNT CFPQLTPGE EPGRLSNFND SLVSRLLADA RTVLGGASAH RTLAGLGKLI ATLRAARSTA QPQPTKQSPL EPPMLDVAEL L TSLLRTES LGLALGQAQE PLHSLLEAAE DLAQELLALR SLVELRALLQ RPRGTSGPLE LLSEALCSVR GPSSTVGPSL NW YEASDLM ELVGQEPESA LPDSSLSPAC SELIGALDSH PLSRLLWRRL KPLILGKLLF APDTPFTRKL MAQVNRTFEE LTL LRDVRE VWEMLGPRIF TFMNDSSNVA MLQRLLQMQD EGRRQPRPGG RDHMEALRSF LDPGSGGYSW QDAHADVGHL VGTL GRVTE CLSLDKLEAA PSEAALVSRA LQLLAEHRFW AGVVFLGPED SSDPTEHPTP DLGPGHVRIK IRMDIDVVTR TNKIR DRFW DPGPAADPLT DLRYVWGGFV YLQDLVERAA VRVLSGANPR AGLYLQQMPY PCYVDDVFLR VLSRSLPLFL TLAWIY SVT LTVKAVVREK ETRLRDTMRA MGLSRAVLWL GWFLSCLGPF LLSAALLVLV LKLGDILPYS HPGVVFLFLA AFAVATV TQ SFLLSAFFSR ANLAAACGGL AYFSLYLPYV LCVAWRDRLP AGGRVAASLL SPVAFGFGCE SLALLEEQGE GAQWHNVG T RPTADVFSLA QVSGLLLLDA ALYGLATWYL EAVCPGQYGI PEPWNFPFRR SYWCGPRPPK SPAPCPTPLD PKVLVEEAP PGLSPGVSVR SLEKRFPGSP QPALRGLSLD FYQGHITAFL GHNGAGKTTT LSILSGLFPP SGGSAFILGH DVRSSMAAIR PHLGVCPQY NVLFDMLTVD EHVWFYGRLK GLSAAVVGPE QDRLLQDVGL VSKQSVQTRH LSGGMQRKLS VAIAFVGGSQ V VILDEPTA GVDPASRRGI WELLLKYREG RTLILSTHHL DEAELLGDRV AVVAGGRLCC CGSPLFLRRH LGSGYYLTLV KA RLPLTTN EKADTDMEGS VDTRQEKKNG SQGSRVGTPQ LLALVQHWVP GARLVEELPH ELVLVLPYTG AHDGSFATLF REL DTRLAE LRLTGYGISD TSLEEIFLKV VEECAADTDM EDGSCGQHLC TGIAGLDVTL RLKMPPQETA LENGEPAGSA PETD QGSGP DAVGRVQGWA LTRQQLQALL LKRFLLARRS RRGLFAQIVL PALFVGLALV FSLIVPPFGH YPALRLSPTM YGAQV SFFS EDAPGDPGRA RLLEALLQEA GLEEPPVQHS SHRFSAPEVP AEVAKVLASG NWTPESPSPA CQCSRPGARR LLPDCP AAA GGPPPPQAVT GSGEVVQNLT GRNLSDFLVK TYPRLVRQGL KTKKWVNEVR YGGFSLGGRD PGLPSGQELG RSVEELW AL LSPLPGGALD RVLKNLTAWA HSLDAQDSLK IWFNNKGWHS MVAFVNRASN AILRAHLPPG PARHAHSITT LNHPLNLT K EQLSEGALMA SSVDVLVSIC VVFAMSFVPA SFTLVLIEER VTRAKHLQLM GGLSPTLYWL GNFLWDMCNY LVPACIVVL IFLAFQQRAY VAPANLPALL LLLLLYGWSI TPLMYPASFF FSVPSTAYVV LTCINLFIGI NGSMATFVLE LFSDQKLQEV SRILKQVFL IFPHFCLGRG LIDMVRNQAM ADAFERLGDR QFQSPLRWEV VGKNLLAMVI QGPLFLLFTL LLQHRSQLLP Q PRVRSLPL LGEEDEDVAR ERERVVQGAT QGDVLVLRNL TKVYRGQRMP AVDRLCLGIP PGECFGLLGV NGAGKTSTFR MV TGDTLAS RGEAVLAGHS VAREPSAAHL SMGYCPQSDA IFELLTGREH LELLARLRGV PEAQVAQTAG SGLARLGLSW YAD RPAGTY SGGNKRKLAT ALALVGDPAV VFLDEPTTGM DPSARRFLWN SLLAVVREGR SVMLTSHSME ECEALCSRLA IMVN GRFRC LGSPQHLKGR FAAGHTLTLR VPAARSQPAA AFVAAEFPGA ELREAHGGRL RFQLPPGGRC ALARVFGELA VHGAE HGVE DFSVSQTMLE EVFLYFSKDQ GKDEDTEEQK EAGVGVDPAP GLQHPKRVSQ FLDDPSTAET VL UniProtKB: Phospholipid-transporting ATPase ABCA7 |
-Macromolecule #4: 2-acetamido-2-deoxy-beta-D-glucopyranose
| Macromolecule | Name: 2-acetamido-2-deoxy-beta-D-glucopyranose / type: ligand / ID: 4 / Number of copies: 4 / Formula: NAG |
|---|---|
| Molecular weight | Theoretical: 221.208 Da |
| Chemical component information |  ChemComp-NAG: |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 0.5 mg/mL |
|---|---|
| Buffer | pH: 7.5 / Details: 25mM HEPES pH 7.5, 150mM NaCl, 0.035% Digitonin |
| Grid | Model: Quantifoil R1.2/1.3 / Support film - Material: CARBON / Support film - topology: HOLEY |
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 90 % / Chamber temperature: 277 K / Instrument: FEI VITROBOT MARK IV |
| Details | Human ABCA7 in Digitonin |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: FEI FALCON III (4k x 4k) / Detector mode: COUNTING / Average exposure time: 60.0 sec. / Average electron dose: 60.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: 2.4 µm / Nominal defocus min: 0.8 µm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller











 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)