[English] 日本語
 Yorodumi
Yorodumi- EMDB-27987: YAR027W and YAR028W in complex with c subunits from yeast VO complex -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | YAR027W and YAR028W in complex with c subunits from yeast VO complex | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
| Biological species |   | |||||||||
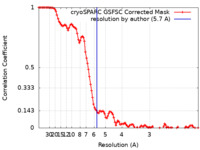
| Method | single particle reconstruction / cryo EM / Resolution: 5.7 Å | |||||||||
 Authors Authors | Wang H / Bueler SA / Rubinstein JL | |||||||||
| Funding support |  Canada, 1 items Canada, 1 items
| |||||||||
 Citation Citation |  Journal: Proc Natl Acad Sci U S A / Year: 2023 Journal: Proc Natl Acad Sci U S A / Year: 2023Title: Structural basis of V-ATPase V region assembly by Vma12p, 21p, and 22p. Authors: Hanlin Wang / Stephanie A Bueler / John L Rubinstein /  Abstract: Vacuolar-type adenosine triphosphatases (V-ATPases) are rotary proton pumps that acidify specific intracellular compartments in almost all eukaryotic cells. These multi-subunit enzymes consist of a ...Vacuolar-type adenosine triphosphatases (V-ATPases) are rotary proton pumps that acidify specific intracellular compartments in almost all eukaryotic cells. These multi-subunit enzymes consist of a soluble catalytic V region and a membrane-embedded proton-translocating V region. V is assembled in the endoplasmic reticulum (ER) membrane, and V is assembled in the cytosol. However, V binds V only after V is transported to the Golgi membrane, thereby preventing acidification of the ER. We isolated V complexes and subcomplexes from bound to V-ATPase assembly factors Vma12p, Vma21p, and Vma22p. Electron cryomicroscopy shows how the Vma12-22p complex recruits subunits a, e, and f to the rotor ring of V while blocking premature binding of V. Vma21p, which contains an ER-retrieval motif, binds the V:Vma12-22p complex, "mature" V, and a complex that appears to contain a ring of loosely packed rotor subunits and the proteins YAR027W and YAR028W. The structures suggest that Vma21p binds assembly intermediates that contain a rotor ring and that activation of proton pumping following assembly of V with V removes Vma21p, allowing V-ATPase to remain in the Golgi. Together, these structures show how Vma12-22p and Vma21p function in V-ATPase assembly and quality control, ensuring the enzyme acidifies only its intended cellular targets. #1:  Journal: Biorxiv / Year: 2022 Journal: Biorxiv / Year: 2022Title: Structural basis of V-ATPase V0 region assembly by Vma12p, 21p, and 22p Authors: Wang H / Bueler SA / Rubinstein JL | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_27987.map.gz emd_27987.map.gz | 118 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-27987-v30.xml emd-27987-v30.xml emd-27987.xml emd-27987.xml | 29.9 KB 29.9 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_27987_fsc.xml emd_27987_fsc.xml | 10.6 KB | Display |  FSC data file FSC data file |
| Images |  emd_27987.png emd_27987.png | 51.2 KB | ||
| Others |  emd_27987_half_map_1.map.gz emd_27987_half_map_1.map.gz emd_27987_half_map_2.map.gz emd_27987_half_map_2.map.gz | 116.2 MB 116.2 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-27987 http://ftp.pdbj.org/pub/emdb/structures/EMD-27987 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-27987 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-27987 | HTTPS FTP |
-Related structure data
| Related structure data |  8eavMC  8easC  8eatC  8eauC M: atomic model generated by this map C: citing same article ( |
|---|
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_27987.map.gz / Format: CCP4 / Size: 125 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_27987.map.gz / Format: CCP4 / Size: 125 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.03 Å | ||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Half map: #2
| File | emd_27987_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: #1
| File | emd_27987_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
+Entire : YAR027W and YAR028W in complex with c subunits from yeast VO complex
+Supramolecule #1: YAR027W and YAR028W in complex with c subunits from yeast VO complex
+Macromolecule #1: YAR027W or YAR028W
+Macromolecule #2: YAR027W or YAR028W
+Macromolecule #3: YAR027W or YAR028W
+Macromolecule #4: YAR027W or YAR028W
+Macromolecule #5: subunit from the c ring of yeast VO complex
+Macromolecule #6: subunit from the c ring of yeast VO complex
+Macromolecule #7: YAR027W or YAR028W
+Macromolecule #8: YAR027W or YAR028W
+Macromolecule #9: YAR027W or YAR028W
+Macromolecule #10: YAR027W or YAR028W
+Macromolecule #11: YAR027W or YAR028W
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.4 |
|---|---|
| Grid | Model: Homemade / Material: COPPER/RHODIUM |
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 80 % / Chamber temperature: 277 K |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: FEI FALCON IV (4k x 4k) / Average electron dose: 49.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: 2.0 µm / Nominal defocus min: 0.5 µm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller







 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)