[English] 日本語
 Yorodumi
Yorodumi- EMDB-27658: cryoEM map of de novo hallucinated homooligomer of C15-C5 symmetr... -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | cryoEM map of de novo hallucinated homooligomer of C15-C5 symmetry, design HALC15-5_262. | ||||||||||||
 Map data Map data | EM map of C15/C5 symmetric hallucinated protein oligomer assembly (HALC15-5_262). | ||||||||||||
 Sample Sample |
| ||||||||||||
 Keywords Keywords | De novo protein design / Computational protein design / Machine learning / Hallucination / homo-oligomers / DE NOVO PROTEIN | ||||||||||||
| Biological species |   | ||||||||||||
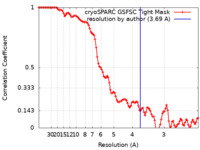
| Method | single particle reconstruction / cryo EM / Resolution: 3.69 Å | ||||||||||||
 Authors Authors | Wicky BIM / Milles LF / Courbet AC / Baker D | ||||||||||||
| Funding support |  United States, 3 items United States, 3 items
| ||||||||||||
 Citation Citation |  Journal: Science / Year: 2022 Journal: Science / Year: 2022Title: Hallucinating symmetric protein assemblies. Authors: B I M Wicky / L F Milles / A Courbet / R J Ragotte / J Dauparas / E Kinfu / S Tipps / R D Kibler / M Baek / F DiMaio / X Li / L Carter / A Kang / H Nguyen / A K Bera / D Baker /  Abstract: Deep learning generative approaches provide an opportunity to broadly explore protein structure space beyond the sequences and structures of natural proteins. Here, we use deep network hallucination ...Deep learning generative approaches provide an opportunity to broadly explore protein structure space beyond the sequences and structures of natural proteins. Here, we use deep network hallucination to generate a wide range of symmetric protein homo-oligomers given only a specification of the number of protomers and the protomer length. Crystal structures of seven designs are very similar to the computational models (median root mean square deviation: 0.6 angstroms), as are three cryo-electron microscopy structures of giant 10-nanometer rings with up to 1550 residues and symmetry; all differ considerably from previously solved structures. Our results highlight the rich diversity of new protein structures that can be generated using deep learning and pave the way for the design of increasingly complex components for nanomachines and biomaterials. #1:  Journal: BioRxiv / Year: 2022 Journal: BioRxiv / Year: 2022Title: Hallucinating protein assemblies Authors: Wicky BIM / Milles LF / Courbet A / Baker D | ||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_27658.map.gz emd_27658.map.gz | 97.1 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-27658-v30.xml emd-27658-v30.xml emd-27658.xml emd-27658.xml | 16.5 KB 16.5 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_27658_fsc.xml emd_27658_fsc.xml | 9.9 KB | Display |  FSC data file FSC data file |
| Images |  emd_27658.png emd_27658.png | 16.9 KB | ||
| Masks |  emd_27658_msk_1.map emd_27658_msk_1.map | 103 MB |  Mask map Mask map | |
| Filedesc metadata |  emd-27658.cif.gz emd-27658.cif.gz | 4.9 KB | ||
| Others |  emd_27658_half_map_1.map.gz emd_27658_half_map_1.map.gz emd_27658_half_map_2.map.gz emd_27658_half_map_2.map.gz | 95.5 MB 95.5 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-27658 http://ftp.pdbj.org/pub/emdb/structures/EMD-27658 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-27658 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-27658 | HTTPS FTP |
-Validation report
| Summary document |  emd_27658_validation.pdf.gz emd_27658_validation.pdf.gz | 719.9 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_27658_full_validation.pdf.gz emd_27658_full_validation.pdf.gz | 719.5 KB | Display | |
| Data in XML |  emd_27658_validation.xml.gz emd_27658_validation.xml.gz | 17.6 KB | Display | |
| Data in CIF |  emd_27658_validation.cif.gz emd_27658_validation.cif.gz | 22.9 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-27658 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-27658 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-27658 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-27658 | HTTPS FTP |
-Related structure data
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_27658.map.gz / Format: CCP4 / Size: 103 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_27658.map.gz / Format: CCP4 / Size: 103 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | EM map of C15/C5 symmetric hallucinated protein oligomer assembly (HALC15-5_262). | ||||||||||||||||||||
| Voxel size | X=Y=Z: 1.16 Å | ||||||||||||||||||||
| Density |
| ||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Mask #1
| File |  emd_27658_msk_1.map emd_27658_msk_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: half map A of C15/C5 symmetric hallucinated protein...
| File | emd_27658_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | half map A of C15/C5 symmetric hallucinated protein oligomer assembly (HALC15-5_262). | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: half map B of C15/C5 symmetric hallucinated protein...
| File | emd_27658_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | half map B of C15/C5 symmetric hallucinated protein oligomer assembly (HALC15-5_262). | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : De novo hallucinated homooligomer of C15-C5 symmetry, design HALC...
| Entire | Name: De novo hallucinated homooligomer of C15-C5 symmetry, design HALC15-5_262. |
|---|---|
| Components |
|
-Supramolecule #1: De novo hallucinated homooligomer of C15-C5 symmetry, design HALC...
| Supramolecule | Name: De novo hallucinated homooligomer of C15-C5 symmetry, design HALC15-5_262. type: complex / ID: 1 / Parent: 0 / Macromolecule list: all |
|---|---|
| Source (natural) | Organism:  |
-Macromolecule #1: HALC15-5_262
| Macromolecule | Name: HALC15-5_262 / type: protein_or_peptide / ID: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Recombinant expression | Organism: Expression vector pET-mod (others) |
| Sequence | String: DVPLTDPKNL NEFLYALGEG LKGMKNLKKL TLTFPSNPLT IPGDISEGFR ELGEGLKGMK NLEELTVTFN DVPLTDPKN LNEFLYALGE GLKGMKNLKK LTLTFPSNPL TIPGDISEGF RELGEGLKGM KNLEELTVTF N DVPLTDPK NLNEFLYALG EGLKGMKNLK ...String: DVPLTDPKNL NEFLYALGEG LKGMKNLKKL TLTFPSNPLT IPGDISEGFR ELGEGLKGMK NLEELTVTFN DVPLTDPKN LNEFLYALGE GLKGMKNLKK LTLTFPSNPL TIPGDISEGF RELGEGLKGM KNLEELTVTF N DVPLTDPK NLNEFLYALG EGLKGMKNLK KLTLTFPSNP LTIPGDISEG FRELGEGLKG MKNLEELTVT FN |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 10 mg/mL | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Buffer | pH: 8.4 Component:
| |||||||||
| Grid | Model: C-flat-2/2 | |||||||||
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 277 K / Instrument: FEI VITROBOT MARK III |
- Electron microscopy
Electron microscopy
| Microscope | TFS GLACIOS |
|---|---|
| Image recording | Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Average electron dose: 50.0 e/Å2 |
| Electron beam | Acceleration voltage: 200 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: 2.5 µm / Nominal defocus min: 1.0 µm |
 Movie
Movie Controller
Controller












 Z
Z Y
Y X
X