+ データを開く
データを開く
- 基本情報
基本情報
| 登録情報 |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| タイトル | Cryo-EM structure of cystinosin in a lumen-open state | |||||||||
 マップデータ マップデータ | Cystinosin Apo Lumen-open | |||||||||
 試料 試料 |
| |||||||||
 キーワード キーワード | Cystine / transporter / lysosome / MEMBRANE PROTEIN / MEMBRANE PROTEIN-Transport protein complex | |||||||||
| 機能・相同性 |  機能・相同性情報 機能・相同性情報regulation of melanin biosynthetic process / solute:proton symporter activity / L-cystine transmembrane transporter activity / L-cystine transport / Transport of inorganic cations/anions and amino acids/oligopeptides / Miscellaneous transport and binding events / regulation of TORC1 signaling / melanin biosynthetic process / grooming behavior / melanosome membrane ...regulation of melanin biosynthetic process / solute:proton symporter activity / L-cystine transmembrane transporter activity / L-cystine transport / Transport of inorganic cations/anions and amino acids/oligopeptides / Miscellaneous transport and binding events / regulation of TORC1 signaling / melanin biosynthetic process / grooming behavior / melanosome membrane / amino acid metabolic process / lens development in camera-type eye / adult walking behavior / long-term memory / ATP metabolic process / monoatomic ion transport / positive regulation of TORC1 signaling / glutathione metabolic process / brain development / visual learning / transmembrane transport / cognition / late endosome / melanosome / protein transport / lysosome / lysosomal membrane / intracellular membrane-bounded organelle / extracellular exosome / plasma membrane 類似検索 - 分子機能 | |||||||||
| 生物種 |  Homo sapiens (ヒト) / Homo sapiens (ヒト) /  | |||||||||
| 手法 | 単粒子再構成法 / クライオ電子顕微鏡法 / 解像度: 3.32 Å | |||||||||
 データ登録者 データ登録者 | Schmiege P / Li X | |||||||||
| 資金援助 |  米国, 2件 米国, 2件
| |||||||||
 引用 引用 |  ジャーナル: Cell / 年: 2022 ジャーナル: Cell / 年: 2022タイトル: Structure and mechanism of human cystine exporter cystinosin. 著者: Xue Guo / Philip Schmiege / Tufa E Assafa / Rong Wang / Yan Xu / Linda Donnelly / Michael Fine / Xiaodan Ni / Jiansen Jiang / Glenn Millhauser / Liang Feng / Xiaochun Li /  要旨: Lysosomal amino acid efflux by proton-driven transporters is essential for lysosomal homeostasis, amino acid recycling, mTOR signaling, and maintaining lysosomal pH. To unravel the mechanisms of ...Lysosomal amino acid efflux by proton-driven transporters is essential for lysosomal homeostasis, amino acid recycling, mTOR signaling, and maintaining lysosomal pH. To unravel the mechanisms of these transporters, we focus on cystinosin, a prototypical lysosomal amino acid transporter that exports cystine to the cytosol, where its reduction to cysteine supplies this limiting amino acid for diverse fundamental processes and controlling nutrient adaptation. Cystinosin mutations cause cystinosis, a devastating lysosomal storage disease. Here, we present structures of human cystinosin in lumen-open, cytosol-open, and cystine-bound states, which uncover the cystine recognition mechanism and capture the key conformational states of the transport cycle. Our structures, along with functional studies and double electron-electron resonance spectroscopic investigations, reveal the molecular basis for the transporter's conformational transitions and protonation switch, show conformation-dependent Ragulator-Rag complex engagement, and demonstrate an unexpected activation mechanism. These findings provide molecular insights into lysosomal amino acid efflux and a potential therapeutic strategy. | |||||||||
| 履歴 |
|
- 構造の表示
構造の表示
| 添付画像 |
|---|
- ダウンロードとリンク
ダウンロードとリンク
-EMDBアーカイブ
| マップデータ |  emd_27489.map.gz emd_27489.map.gz | 97.1 MB |  EMDBマップデータ形式 EMDBマップデータ形式 | |
|---|---|---|---|---|
| ヘッダ (付随情報) |  emd-27489-v30.xml emd-27489-v30.xml emd-27489.xml emd-27489.xml | 17 KB 17 KB | 表示 表示 |  EMDBヘッダ EMDBヘッダ |
| 画像 |  emd_27489.png emd_27489.png | 82.5 KB | ||
| Filedesc metadata |  emd-27489.cif.gz emd-27489.cif.gz | 5.7 KB | ||
| その他 |  emd_27489_half_map_1.map.gz emd_27489_half_map_1.map.gz emd_27489_half_map_2.map.gz emd_27489_half_map_2.map.gz | 95.8 MB 95.8 MB | ||
| アーカイブディレクトリ |  http://ftp.pdbj.org/pub/emdb/structures/EMD-27489 http://ftp.pdbj.org/pub/emdb/structures/EMD-27489 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-27489 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-27489 | HTTPS FTP |
-検証レポート
| 文書・要旨 |  emd_27489_validation.pdf.gz emd_27489_validation.pdf.gz | 804 KB | 表示 |  EMDB検証レポート EMDB検証レポート |
|---|---|---|---|---|
| 文書・詳細版 |  emd_27489_full_validation.pdf.gz emd_27489_full_validation.pdf.gz | 803.6 KB | 表示 | |
| XML形式データ |  emd_27489_validation.xml.gz emd_27489_validation.xml.gz | 13.4 KB | 表示 | |
| CIF形式データ |  emd_27489_validation.cif.gz emd_27489_validation.cif.gz | 15.8 KB | 表示 | |
| アーカイブディレクトリ |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-27489 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-27489 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-27489 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-27489 | HTTPS FTP |
-関連構造データ
- リンク
リンク
| EMDBのページ |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| 「今月の分子」の関連する項目 |
- マップ
マップ
| ファイル |  ダウンロード / ファイル: emd_27489.map.gz / 形式: CCP4 / 大きさ: 103 MB / タイプ: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) ダウンロード / ファイル: emd_27489.map.gz / 形式: CCP4 / 大きさ: 103 MB / タイプ: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 注釈 | Cystinosin Apo Lumen-open | ||||||||||||||||||||||||||||||||||||
| 投影像・断面図 | 画像のコントロール
画像は Spider により作成 | ||||||||||||||||||||||||||||||||||||
| ボクセルのサイズ | X=Y=Z: 0.844 Å | ||||||||||||||||||||||||||||||||||||
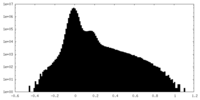
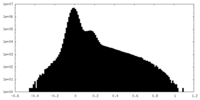
| 密度 |
| ||||||||||||||||||||||||||||||||||||
| 対称性 | 空間群: 1 | ||||||||||||||||||||||||||||||||||||
| 詳細 | EMDB XML:
|
-添付データ
-ハーフマップ: Half Map 1
| ファイル | emd_27489_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
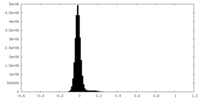
| 注釈 | Half Map 1 | ||||||||||||
| 投影像・断面図 |
| ||||||||||||
| 密度ヒストグラム |
-ハーフマップ: Half Map 2
| ファイル | emd_27489_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
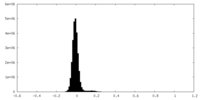
| 注釈 | Half Map 2 | ||||||||||||
| 投影像・断面図 |
| ||||||||||||
| 密度ヒストグラム |
- 試料の構成要素
試料の構成要素
-全体 : Cystinosin-Fab 3H5 complex in lumen-open conformation
| 全体 | 名称: Cystinosin-Fab 3H5 complex in lumen-open conformation |
|---|---|
| 要素 |
|
-超分子 #1: Cystinosin-Fab 3H5 complex in lumen-open conformation
| 超分子 | 名称: Cystinosin-Fab 3H5 complex in lumen-open conformation タイプ: complex / ID: 1 / 親要素: 0 / 含まれる分子: all |
|---|
-超分子 #2: Cystinosin
| 超分子 | 名称: Cystinosin / タイプ: complex / ID: 2 / 親要素: 1 / 含まれる分子: #1 |
|---|---|
| 由来(天然) | 生物種:  Homo sapiens (ヒト) Homo sapiens (ヒト) |
-超分子 #3: Fab 3H5
| 超分子 | 名称: Fab 3H5 / タイプ: complex / ID: 3 / 親要素: 1 / 含まれる分子: #2-#3 |
|---|---|
| 由来(天然) | 生物種:  |
-分子 #1: Isoform 2 of Cystinosin
| 分子 | 名称: Isoform 2 of Cystinosin / タイプ: protein_or_peptide / ID: 1 / コピー数: 1 / 光学異性体: LEVO |
|---|---|
| 由来(天然) | 生物種:  Homo sapiens (ヒト) Homo sapiens (ヒト) |
| 分子量 | 理論値: 46.080355 KDa |
| 組換発現 | 生物種:  Homo sapiens (ヒト) Homo sapiens (ヒト) |
| 配列 | 文字列: MIRNWLTIFI LFPLKLVEKC ESSVSLTVPP VVKLENGSST NVSLTLRPPL NATLVITFEI TFRSKNITIL ELPDEVVVPP GVTNSSFQV TSQNVGQLTV YLHGNHSNQT GPRIRFLVIR SSAISIINQV IGWIYFVAWS ISFYPQVIMN WRRKSVIGLS F DFVALNLT ...文字列: MIRNWLTIFI LFPLKLVEKC ESSVSLTVPP VVKLENGSST NVSLTLRPPL NATLVITFEI TFRSKNITIL ELPDEVVVPP GVTNSSFQV TSQNVGQLTV YLHGNHSNQT GPRIRFLVIR SSAISIINQV IGWIYFVAWS ISFYPQVIMN WRRKSVIGLS F DFVALNLT GFVAYSVFNI GLLWVPYIKE QFLLKYPNGV NPVNSNDVFF SLHAVVLTLI IIVQCCLYER GGQRVSWPAI GF LVLAWLF AFVTMIVAAV GVITWLQFLF CFSYIKLAVT LVKYFPQAYM NFYYKSTEGW SIGNVLLDFT GGSFSLLQMF LQS YNNDQW TLIFGDPTKF GLGVFSIVFD VVFFIQHFCL YRKRPGLQAA RTGSGSRLRQ DWAPSLQPKA LPQTTSVSAS SLKG DYKDD DDK UniProtKB: Cystinosin |
-分子 #2: Fab 3H5 Heavy Chain
| 分子 | 名称: Fab 3H5 Heavy Chain / タイプ: protein_or_peptide / ID: 2 / コピー数: 1 / 光学異性体: LEVO |
|---|---|
| 由来(天然) | 生物種:  |
| 分子量 | 理論値: 26.768107 KDa |
| 組換発現 | 生物種:  Homo sapiens (ヒト) Homo sapiens (ヒト) |
| 配列 | 文字列: MGWSCIILFL VATATGVHSE VMLVESGGGL VKPGGSLKLS CAASGFTFSN YAMSWVRQTP EKRLEWVAAI SGNEGTYTYY PDSVRGRFT ISRDNARNNL YLQISSLRSE DTALYYCARY GLVGALDFWG QGASVTVSSA STKGPSVFPL APSSKSTSGG T AALGCLVK ...文字列: MGWSCIILFL VATATGVHSE VMLVESGGGL VKPGGSLKLS CAASGFTFSN YAMSWVRQTP EKRLEWVAAI SGNEGTYTYY PDSVRGRFT ISRDNARNNL YLQISSLRSE DTALYYCARY GLVGALDFWG QGASVTVSSA STKGPSVFPL APSSKSTSGG T AALGCLVK DYFPEPVTVS WNSGALTSGV HTFPAVLQSS GLYSLSSVVT VPSSSLGTQT YICNVNHKPS NTKVDKRVEP KS CDKTHHH HHH |
-分子 #3: Fab 3H5 Kappa chain
| 分子 | 名称: Fab 3H5 Kappa chain / タイプ: protein_or_peptide / ID: 3 / コピー数: 1 / 光学異性体: LEVO |
|---|---|
| 由来(天然) | 生物種:  |
| 分子量 | 理論値: 25.468404 KDa |
| 組換発現 | 生物種:  Homo sapiens (ヒト) Homo sapiens (ヒト) |
| 配列 | 文字列: MGWSCIILFL VATATGVHSD IQMNQSPSTL SASLGDTITI TCRASQNIDV WLNWYQQKPG DIPKLLIYEA SNLHTGVPSR FSGSGSGTD FTLAISSLQP EDIATYYCLQ GQDYPFTFGS GTKLEIKRTV AAPSVFIFPP SDEQLKSGTA SVVCLLNNFY P REAKVQWK ...文字列: MGWSCIILFL VATATGVHSD IQMNQSPSTL SASLGDTITI TCRASQNIDV WLNWYQQKPG DIPKLLIYEA SNLHTGVPSR FSGSGSGTD FTLAISSLQP EDIATYYCLQ GQDYPFTFGS GTKLEIKRTV AAPSVFIFPP SDEQLKSGTA SVVCLLNNFY P REAKVQWK VDNALQSGNS QESVTEQDSK DSTYSLSSTL TLSKADYEKH KVYACEVTHQ GLSSPVTKSF NRGEC |
-実験情報
-構造解析
| 手法 | クライオ電子顕微鏡法 |
|---|---|
 解析 解析 | 単粒子再構成法 |
| 試料の集合状態 | particle |
- 試料調製
試料調製
| 緩衝液 | pH: 7.5 |
|---|---|
| 凍結 | 凍結剤: ETHANE |
- 電子顕微鏡法
電子顕微鏡法
| 顕微鏡 | FEI TITAN KRIOS |
|---|---|
| 撮影 | フィルム・検出器のモデル: GATAN K3 (6k x 4k) / 平均電子線量: 61.5 e/Å2 |
| 電子線 | 加速電圧: 300 kV / 電子線源:  FIELD EMISSION GUN FIELD EMISSION GUN |
| 電子光学系 | 照射モード: FLOOD BEAM / 撮影モード: BRIGHT FIELD / 最大 デフォーカス(公称値): 2.0 µm / 最小 デフォーカス(公称値): 1.0 µm |
| 実験機器 |  モデル: Titan Krios / 画像提供: FEI Company |
- 画像解析
画像解析
| 初期モデル | モデルのタイプ: OTHER |
|---|---|
| 最終 再構成 | 解像度のタイプ: BY AUTHOR / 解像度: 3.32 Å / 解像度の算出法: FSC 0.143 CUT-OFF / 使用した粒子像数: 310223 |
| 初期 角度割当 | タイプ: NOT APPLICABLE |
| 最終 角度割当 | タイプ: NOT APPLICABLE |
 ムービー
ムービー コントローラー
コントローラー















 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)