+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo-EM structure of cystinosin in a lumen-open state | |||||||||
 Map data Map data | Cystinosin Apo Lumen-open | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | Cystine / transporter / lysosome / MEMBRANE PROTEIN / MEMBRANE PROTEIN-Transport protein complex | |||||||||
| Function / homology |  Function and homology information Function and homology informationregulation of melanin biosynthetic process / solute:proton symporter activity / L-cystine transmembrane transporter activity / L-cystine transport / : / Miscellaneous transport and binding events / regulation of TORC1 signaling / melanin biosynthetic process / grooming behavior / melanosome membrane ...regulation of melanin biosynthetic process / solute:proton symporter activity / L-cystine transmembrane transporter activity / L-cystine transport / : / Miscellaneous transport and binding events / regulation of TORC1 signaling / melanin biosynthetic process / grooming behavior / melanosome membrane / amino acid metabolic process / lens development in camera-type eye / adult walking behavior / long-term memory / ATP metabolic process / monoatomic ion transport / glutathione metabolic process / positive regulation of TORC1 signaling / brain development / visual learning / transmembrane transport / cognition / melanosome / late endosome / protein transport / lysosome / intracellular membrane-bounded organelle / lysosomal membrane / extracellular exosome / plasma membrane Similarity search - Function | |||||||||
| Biological species |  Homo sapiens (human) / Homo sapiens (human) /  | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 3.32 Å | |||||||||
 Authors Authors | Schmiege P / Li X | |||||||||
| Funding support |  United States, 2 items United States, 2 items
| |||||||||
 Citation Citation |  Journal: Cell / Year: 2022 Journal: Cell / Year: 2022Title: Structure and mechanism of human cystine exporter cystinosin. Authors: Xue Guo / Philip Schmiege / Tufa E Assafa / Rong Wang / Yan Xu / Linda Donnelly / Michael Fine / Xiaodan Ni / Jiansen Jiang / Glenn Millhauser / Liang Feng / Xiaochun Li /  Abstract: Lysosomal amino acid efflux by proton-driven transporters is essential for lysosomal homeostasis, amino acid recycling, mTOR signaling, and maintaining lysosomal pH. To unravel the mechanisms of ...Lysosomal amino acid efflux by proton-driven transporters is essential for lysosomal homeostasis, amino acid recycling, mTOR signaling, and maintaining lysosomal pH. To unravel the mechanisms of these transporters, we focus on cystinosin, a prototypical lysosomal amino acid transporter that exports cystine to the cytosol, where its reduction to cysteine supplies this limiting amino acid for diverse fundamental processes and controlling nutrient adaptation. Cystinosin mutations cause cystinosis, a devastating lysosomal storage disease. Here, we present structures of human cystinosin in lumen-open, cytosol-open, and cystine-bound states, which uncover the cystine recognition mechanism and capture the key conformational states of the transport cycle. Our structures, along with functional studies and double electron-electron resonance spectroscopic investigations, reveal the molecular basis for the transporter's conformational transitions and protonation switch, show conformation-dependent Ragulator-Rag complex engagement, and demonstrate an unexpected activation mechanism. These findings provide molecular insights into lysosomal amino acid efflux and a potential therapeutic strategy. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_27489.map.gz emd_27489.map.gz | 97.1 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-27489-v30.xml emd-27489-v30.xml emd-27489.xml emd-27489.xml | 17 KB 17 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_27489.png emd_27489.png | 82.5 KB | ||
| Filedesc metadata |  emd-27489.cif.gz emd-27489.cif.gz | 5.7 KB | ||
| Others |  emd_27489_half_map_1.map.gz emd_27489_half_map_1.map.gz emd_27489_half_map_2.map.gz emd_27489_half_map_2.map.gz | 95.8 MB 95.8 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-27489 http://ftp.pdbj.org/pub/emdb/structures/EMD-27489 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-27489 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-27489 | HTTPS FTP |
-Related structure data
| Related structure data |  8dkiMC  8dkeC  8dkmC  8dkwC  8dkxC  8dypC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_27489.map.gz / Format: CCP4 / Size: 103 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_27489.map.gz / Format: CCP4 / Size: 103 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Cystinosin Apo Lumen-open | ||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.844 Å | ||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Half map: Half Map 1
| File | emd_27489_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Half Map 1 | ||||||||||||
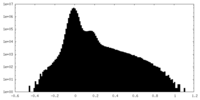
| Projections & Slices |
| ||||||||||||
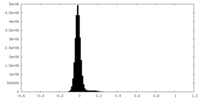
| Density Histograms |
-Half map: Half Map 2
| File | emd_27489_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Half Map 2 | ||||||||||||
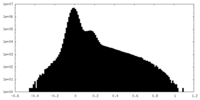
| Projections & Slices |
| ||||||||||||
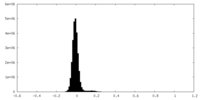
| Density Histograms |
- Sample components
Sample components
-Entire : Cystinosin-Fab 3H5 complex in lumen-open conformation
| Entire | Name: Cystinosin-Fab 3H5 complex in lumen-open conformation |
|---|---|
| Components |
|
-Supramolecule #1: Cystinosin-Fab 3H5 complex in lumen-open conformation
| Supramolecule | Name: Cystinosin-Fab 3H5 complex in lumen-open conformation / type: complex / ID: 1 / Parent: 0 / Macromolecule list: all |
|---|
-Supramolecule #2: Cystinosin
| Supramolecule | Name: Cystinosin / type: complex / ID: 2 / Parent: 1 / Macromolecule list: #1 |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
-Supramolecule #3: Fab 3H5
| Supramolecule | Name: Fab 3H5 / type: complex / ID: 3 / Parent: 1 / Macromolecule list: #2-#3 |
|---|---|
| Source (natural) | Organism:  |
-Macromolecule #1: Isoform 2 of Cystinosin
| Macromolecule | Name: Isoform 2 of Cystinosin / type: protein_or_peptide / ID: 1 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 46.080355 KDa |
| Recombinant expression | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: MIRNWLTIFI LFPLKLVEKC ESSVSLTVPP VVKLENGSST NVSLTLRPPL NATLVITFEI TFRSKNITIL ELPDEVVVPP GVTNSSFQV TSQNVGQLTV YLHGNHSNQT GPRIRFLVIR SSAISIINQV IGWIYFVAWS ISFYPQVIMN WRRKSVIGLS F DFVALNLT ...String: MIRNWLTIFI LFPLKLVEKC ESSVSLTVPP VVKLENGSST NVSLTLRPPL NATLVITFEI TFRSKNITIL ELPDEVVVPP GVTNSSFQV TSQNVGQLTV YLHGNHSNQT GPRIRFLVIR SSAISIINQV IGWIYFVAWS ISFYPQVIMN WRRKSVIGLS F DFVALNLT GFVAYSVFNI GLLWVPYIKE QFLLKYPNGV NPVNSNDVFF SLHAVVLTLI IIVQCCLYER GGQRVSWPAI GF LVLAWLF AFVTMIVAAV GVITWLQFLF CFSYIKLAVT LVKYFPQAYM NFYYKSTEGW SIGNVLLDFT GGSFSLLQMF LQS YNNDQW TLIFGDPTKF GLGVFSIVFD VVFFIQHFCL YRKRPGLQAA RTGSGSRLRQ DWAPSLQPKA LPQTTSVSAS SLKG DYKDD DDK UniProtKB: Cystinosin |
-Macromolecule #2: Fab 3H5 Heavy Chain
| Macromolecule | Name: Fab 3H5 Heavy Chain / type: protein_or_peptide / ID: 2 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 26.768107 KDa |
| Recombinant expression | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: MGWSCIILFL VATATGVHSE VMLVESGGGL VKPGGSLKLS CAASGFTFSN YAMSWVRQTP EKRLEWVAAI SGNEGTYTYY PDSVRGRFT ISRDNARNNL YLQISSLRSE DTALYYCARY GLVGALDFWG QGASVTVSSA STKGPSVFPL APSSKSTSGG T AALGCLVK ...String: MGWSCIILFL VATATGVHSE VMLVESGGGL VKPGGSLKLS CAASGFTFSN YAMSWVRQTP EKRLEWVAAI SGNEGTYTYY PDSVRGRFT ISRDNARNNL YLQISSLRSE DTALYYCARY GLVGALDFWG QGASVTVSSA STKGPSVFPL APSSKSTSGG T AALGCLVK DYFPEPVTVS WNSGALTSGV HTFPAVLQSS GLYSLSSVVT VPSSSLGTQT YICNVNHKPS NTKVDKRVEP KS CDKTHHH HHH |
-Macromolecule #3: Fab 3H5 Kappa chain
| Macromolecule | Name: Fab 3H5 Kappa chain / type: protein_or_peptide / ID: 3 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 25.468404 KDa |
| Recombinant expression | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: MGWSCIILFL VATATGVHSD IQMNQSPSTL SASLGDTITI TCRASQNIDV WLNWYQQKPG DIPKLLIYEA SNLHTGVPSR FSGSGSGTD FTLAISSLQP EDIATYYCLQ GQDYPFTFGS GTKLEIKRTV AAPSVFIFPP SDEQLKSGTA SVVCLLNNFY P REAKVQWK ...String: MGWSCIILFL VATATGVHSD IQMNQSPSTL SASLGDTITI TCRASQNIDV WLNWYQQKPG DIPKLLIYEA SNLHTGVPSR FSGSGSGTD FTLAISSLQP EDIATYYCLQ GQDYPFTFGS GTKLEIKRTV AAPSVFIFPP SDEQLKSGTA SVVCLLNNFY P REAKVQWK VDNALQSGNS QESVTEQDSK DSTYSLSSTL TLSKADYEKH KVYACEVTHQ GLSSPVTKSF NRGEC |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.5 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Average electron dose: 61.5 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: 2.0 µm / Nominal defocus min: 1.0 µm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
- Image processing
Image processing
| Startup model | Type of model: OTHER |
|---|---|
| Final reconstruction | Resolution.type: BY AUTHOR / Resolution: 3.32 Å / Resolution method: FSC 0.143 CUT-OFF / Number images used: 310223 |
| Initial angle assignment | Type: NOT APPLICABLE |
| Final angle assignment | Type: NOT APPLICABLE |
 Movie
Movie Controller
Controller









 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)