+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo-EM structure of BCL10 CARD - MALT1 DD filament | |||||||||
 Map data Map data | CryoEM structure BCL10 CARD-MALT1 | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | filament / CBM complex / IMMUNE SYSTEM | |||||||||
| Function / homology |  Function and homology information Function and homology informationpositive regulation of lymphotoxin A production / polkadots / B-1 B cell differentiation / positive regulation of T-helper 17 cell differentiation / CBM complex / regulation of T cell receptor signaling pathway / protein kinase B binding / antifungal innate immune response / response to fungus / CARD domain binding ...positive regulation of lymphotoxin A production / polkadots / B-1 B cell differentiation / positive regulation of T-helper 17 cell differentiation / CBM complex / regulation of T cell receptor signaling pathway / protein kinase B binding / antifungal innate immune response / response to fungus / CARD domain binding / positive regulation of mast cell cytokine production / T cell apoptotic process / negative regulation of mature B cell apoptotic process / B cell apoptotic process / CLEC7A/inflammasome pathway / programmed cell death / nuclear export / non-canonical NF-kappaB signal transduction / positive regulation of extrinsic apoptotic signaling pathway / response to food / toll-like receptor signaling pathway / small molecule binding / positive regulation of T cell receptor signaling pathway / immunoglobulin mediated immune response / B cell activation / immunological synapse / general transcription initiation factor binding / positive regulation of phosphorylation / NF-kappaB binding / cellular defense response / endopeptidase activator activity / T cell proliferation / cytoplasmic microtubule / signaling adaptor activity / positive regulation of interleukin-2 production / : / lipopolysaccharide-mediated signaling pathway / positive regulation of protein ubiquitination / positive regulation of interleukin-1 beta production / positive regulation of interleukin-8 production / neural tube closure / apoptotic signaling pathway / Activation of NF-kappaB in B cells / cellular response to mechanical stimulus / defense response / : / positive regulation of T cell cytokine production / protein homooligomerization / positive regulation of T cell activation / positive regulation of interleukin-6 production / CLEC7A (Dectin-1) signaling / FCERI mediated NF-kB activation / fibrillar center / ubiquitin-protein transferase activity / Downstream TCR signaling / peptidase activity / T cell receptor signaling pathway / E3 ubiquitin ligases ubiquitinate target proteins / cellular response to lipopolysaccharide / protease binding / endopeptidase activity / regulation of apoptotic process / protein-macromolecule adaptor activity / adaptive immune response / transcription coactivator activity / Hydrolases; Acting on peptide bonds (peptidases); Cysteine endopeptidases / lysosome / positive regulation of canonical NF-kappaB signal transduction / positive regulation of apoptotic process / membrane raft / innate immune response / cysteine-type endopeptidase activity / ubiquitin protein ligase binding / negative regulation of apoptotic process / positive regulation of DNA-templated transcription / protein-containing complex binding / perinuclear region of cytoplasm / protein-containing complex / proteolysis / nucleoplasm / identical protein binding / nucleus / cytosol / cytoplasm Similarity search - Function | |||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | |||||||||
| Method | helical reconstruction / cryo EM / Resolution: 4.3 Å | |||||||||
 Authors Authors | David L / Wu H | |||||||||
| Funding support | 1 items
| |||||||||
 Citation Citation |  Journal: Cancer Discov / Year: 2022 Journal: Cancer Discov / Year: 2022Title: BCL10 Mutations Define Distinct Dependencies Guiding Precision Therapy for DLBCL. Authors: Min Xia / Liron David / Matt Teater / Johana Gutierrez / Xiang Wang / Cem Meydan / Andrew Lytle / Graham W Slack / David W Scott / Ryan D Morin / Ozlem Onder / Kojo S J Elenitoba-Johnson / ...Authors: Min Xia / Liron David / Matt Teater / Johana Gutierrez / Xiang Wang / Cem Meydan / Andrew Lytle / Graham W Slack / David W Scott / Ryan D Morin / Ozlem Onder / Kojo S J Elenitoba-Johnson / Nahuel Zamponi / Leandro Cerchietti / Tianbao Lu / Ulrike Philippar / Lorena Fontan / Hao Wu / Ari M Melnick /    Abstract: Activated B cell-like diffuse large B-cell lymphomas (ABC-DLBCL) have unfavorable outcomes and chronic activation of CARD11-BCL10-MALT1 (CBM) signal amplification complexes that form due to ...Activated B cell-like diffuse large B-cell lymphomas (ABC-DLBCL) have unfavorable outcomes and chronic activation of CARD11-BCL10-MALT1 (CBM) signal amplification complexes that form due to polymerization of BCL10 subunits, which is affected by recurrent somatic mutations in ABC-DLBCLs. Herein, we show that BCL10 mutants fall into at least two functionally distinct classes: missense mutations of the BCL10 CARD domain and truncation of its C-terminal tail. Truncating mutations abrogated a motif through which MALT1 inhibits BCL10 polymerization, trapping MALT1 in its activated filament-bound state. CARD missense mutations enhanced BCL10 filament formation, forming glutamine network structures that stabilize BCL10 filaments. Mutant forms of BCL10 were less dependent on upstream CARD11 activation and thus manifested resistance to BTK inhibitors, whereas BCL10 truncating but not CARD mutants were hypersensitive to MALT1 inhibitors. Therefore, BCL10 mutations are potential biomarkers for BTK inhibitor resistance in ABC-DLBCL, and further precision can be achieved by selecting therapy based on specific biochemical effects of distinct mutation classes. SIGNIFICANCE: ABC-DLBCLs feature frequent mutations of signaling mediators that converge on the CBM complex. We use structure-function approaches to reveal that BCL10 mutations fall into two distinct ...SIGNIFICANCE: ABC-DLBCLs feature frequent mutations of signaling mediators that converge on the CBM complex. We use structure-function approaches to reveal that BCL10 mutations fall into two distinct biochemical classes. Both classes confer resistance to BTK inhibitors, whereas BCL10 truncations confer hyperresponsiveness to MALT1 inhibitors, providing a road map for precision therapies in ABC-DLBCLs. See related commentary by Phelan and Oellerich, p. 1844. This article is highlighted in the In This Issue feature, p. 1825. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_27100.map.gz emd_27100.map.gz | 118.9 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-27100-v30.xml emd-27100-v30.xml emd-27100.xml emd-27100.xml | 16.2 KB 16.2 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_27100.png emd_27100.png | 173.3 KB | ||
| Filedesc metadata |  emd-27100.cif.gz emd-27100.cif.gz | 5.4 KB | ||
| Others |  emd_27100_half_map_1.map.gz emd_27100_half_map_1.map.gz emd_27100_half_map_2.map.gz emd_27100_half_map_2.map.gz | 108.1 MB 108.1 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-27100 http://ftp.pdbj.org/pub/emdb/structures/EMD-27100 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-27100 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-27100 | HTTPS FTP |
-Validation report
| Summary document |  emd_27100_validation.pdf.gz emd_27100_validation.pdf.gz | 940.8 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_27100_full_validation.pdf.gz emd_27100_full_validation.pdf.gz | 940.4 KB | Display | |
| Data in XML |  emd_27100_validation.xml.gz emd_27100_validation.xml.gz | 14.3 KB | Display | |
| Data in CIF |  emd_27100_validation.cif.gz emd_27100_validation.cif.gz | 16.9 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-27100 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-27100 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-27100 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-27100 | HTTPS FTP |
-Related structure data
| Related structure data |  8czoMC  8czdC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_27100.map.gz / Format: CCP4 / Size: 137.1 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_27100.map.gz / Format: CCP4 / Size: 137.1 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | CryoEM structure BCL10 CARD-MALT1 | ||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.1019 Å | ||||||||||||||||||||||||||||||||||||
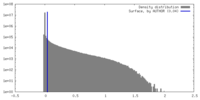
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Half map: half2 map
| File | emd_27100_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | half2 map | ||||||||||||
| Projections & Slices |
| ||||||||||||
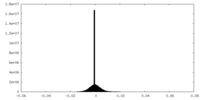
| Density Histograms |
-Half map: half1 map
| File | emd_27100_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | half1 map | ||||||||||||
| Projections & Slices |
| ||||||||||||
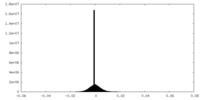
| Density Histograms |
- Sample components
Sample components
-Entire : filament
| Entire | Name: filament |
|---|---|
| Components |
|
-Supramolecule #1: filament
| Supramolecule | Name: filament / type: complex / ID: 1 / Parent: 0 / Macromolecule list: all |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
-Macromolecule #1: Mucosa-associated lymphoid tissue lymphoma translocation protein 1
| Macromolecule | Name: Mucosa-associated lymphoid tissue lymphoma translocation protein 1 type: protein_or_peptide / ID: 1 / Number of copies: 22 / Enantiomer: LEVO EC number: Hydrolases; Acting on peptide bonds (peptidases); Cysteine endopeptidases |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 10.615389 KDa |
| Recombinant expression | Organism:  Escherichia phage EcSzw-2 (virus) Escherichia phage EcSzw-2 (virus) |
| Sequence | String: TLNRLREPLL RRLSELLDQA PEGRGWRRLA ELAGSRGRLR LSCLDLEQCS LKVLEPEGSP SLCLLKLMGE KGCTVTELSD FLQAMEHTE VLQLL UniProtKB: Mucosa-associated lymphoid tissue lymphoma translocation protein 1 |
-Macromolecule #2: B-cell lymphoma/leukemia 10
| Macromolecule | Name: B-cell lymphoma/leukemia 10 / type: protein_or_peptide / ID: 2 / Number of copies: 22 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 12.614566 KDa |
| Recombinant expression | Organism:  Escherichia phage EcSzw-2 (virus) Escherichia phage EcSzw-2 (virus) |
| Sequence | String: EEDLTEVKKD ALENLRVYLC EKIIAERHFD HLRAKKILSR EDTEEISCRT SSRKRAGKLL DYLQENPKGL DTLVESIRRE KTQNFLIQK ITDEVLKLRN IKLEHLK UniProtKB: B-cell lymphoma/leukemia 10 |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | helical reconstruction |
| Aggregation state | filament |
- Sample preparation
Sample preparation
| Buffer | pH: 7.5 Component:
| |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Instrument: FEI VITROBOT MARK III |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: FEI FALCON II (4k x 4k) / Number grids imaged: 1 / Number real images: 700 / Average electron dose: 55.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | C2 aperture diameter: 2.7 µm / Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: 2.5 µm / Nominal defocus min: 1.0 µm / Nominal magnification: 47000 |
| Sample stage | Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
- Image processing
Image processing
| Final reconstruction | Applied symmetry - Helical parameters - Δz: 5.0 Å Applied symmetry - Helical parameters - Δ&Phi: -100.8 ° Applied symmetry - Helical parameters - Axial symmetry: C1 (asymmetric) Resolution.type: BY AUTHOR / Resolution: 4.3 Å / Resolution method: FSC 0.143 CUT-OFF / Software - Name: RELION (ver. 3.1) / Number images used: 23000 |
|---|---|
| Startup model | Type of model: EMDB MAP EMDB ID: |
| Final angle assignment | Type: NOT APPLICABLE |
 Movie
Movie Controller
Controller














 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)