[English] 日本語
 Yorodumi
Yorodumi- EMDB-26916: Local refinement of RhAG-RhCE-ANK1(AR1-5), from consensus refinem... -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Local refinement of RhAG-RhCE-ANK1(AR1-5), from consensus refinement of all classes | |||||||||
 Map data Map data | Main map used for model building/refinement. Density modified and cropped using phenix.resolve_cryo_em, resampled on fine grid using relion_image_handler. | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | Membrane Protein / Anion Exchange / Erythrocyte / Glycoprotein / TRANSPORT PROTEIN | |||||||||
| Function / homology |  Function and homology information Function and homology informationmethylammonium transmembrane transport / Defective RHAG causes regulator type Rh-null hemolytic anemia (RHN) / Rhesus blood group biosynthesis / methylammonium transmembrane transporter activity / Rhesus glycoproteins mediate ammonium transport / carbon dioxide transmembrane transport / carbon dioxide transmembrane transporter activity / ammonium homeostasis / spectrin-associated cytoskeleton / positive regulation of organelle organization ...methylammonium transmembrane transport / Defective RHAG causes regulator type Rh-null hemolytic anemia (RHN) / Rhesus blood group biosynthesis / methylammonium transmembrane transporter activity / Rhesus glycoproteins mediate ammonium transport / carbon dioxide transmembrane transport / carbon dioxide transmembrane transporter activity / ammonium homeostasis / spectrin-associated cytoskeleton / positive regulation of organelle organization / maintenance of epithelial cell apical/basal polarity / NrCAM interactions / Neurofascin interactions / leak channel activity / ammonium transmembrane transport / intracellular monoatomic ion homeostasis / ammonium channel activity / ankyrin-1 complex / CHL1 interactions / cytoskeletal anchor activity / bicarbonate transport / M band / : / Interaction between L1 and Ankyrins / ankyrin binding / spectrin binding / exocytosis / axolemma / endoplasmic reticulum to Golgi vesicle-mediated transport / erythrocyte development / COPI-mediated anterograde transport / cytoskeleton organization / sarcoplasmic reticulum / protein localization to plasma membrane / carbon dioxide transport / Erythrocytes take up oxygen and release carbon dioxide / Erythrocytes take up carbon dioxide and release oxygen / sarcolemma / structural constituent of cytoskeleton / multicellular organismal-level iron ion homeostasis / cytoplasmic side of plasma membrane / Z disc / ATPase binding / protein phosphatase binding / basolateral plasma membrane / transmembrane transporter binding / cytoskeleton / postsynaptic membrane / neuron projection / structural molecule activity / enzyme binding / signal transduction / membrane / plasma membrane / cytosol Similarity search - Function | |||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | |||||||||
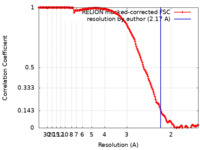
| Method | single particle reconstruction / cryo EM / Resolution: 2.17 Å | |||||||||
 Authors Authors | Vallese F / Kim K / Yen LY / Johnston JD / Noble AJ / Cali T / Clarke OB | |||||||||
| Funding support | 1 items
| |||||||||
 Citation Citation |  Journal: Nat Struct Mol Biol / Year: 2022 Journal: Nat Struct Mol Biol / Year: 2022Title: Architecture of the human erythrocyte ankyrin-1 complex. Authors: Francesca Vallese / Kookjoo Kim / Laura Y Yen / Jake D Johnston / Alex J Noble / Tito Calì / Oliver Biggs Clarke /   Abstract: The stability and shape of the erythrocyte membrane is provided by the ankyrin-1 complex, but how it tethers the spectrin-actin cytoskeleton to the lipid bilayer and the nature of its association ...The stability and shape of the erythrocyte membrane is provided by the ankyrin-1 complex, but how it tethers the spectrin-actin cytoskeleton to the lipid bilayer and the nature of its association with the band 3 anion exchanger and the Rhesus glycoproteins remains unknown. Here we present structures of ankyrin-1 complexes purified from human erythrocytes. We reveal the architecture of a core complex of ankyrin-1, the Rhesus proteins RhAG and RhCE, the band 3 anion exchanger, protein 4.2, glycophorin A and glycophorin B. The distinct T-shaped conformation of membrane-bound ankyrin-1 facilitates recognition of RhCE and, unexpectedly, the water channel aquaporin-1. Together, our results uncover the molecular details of ankyrin-1 association with the erythrocyte membrane, and illustrate the mechanism of ankyrin-mediated membrane protein clustering. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_26916.map.gz emd_26916.map.gz | 66.8 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-26916-v30.xml emd-26916-v30.xml emd-26916.xml emd-26916.xml | 34.7 KB 34.7 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_26916_fsc.xml emd_26916_fsc.xml | 15.8 KB | Display |  FSC data file FSC data file |
| Images |  emd_26916.png emd_26916.png | 67.6 KB | ||
| Filedesc metadata |  emd-26916.cif.gz emd-26916.cif.gz | 7 KB | ||
| Others |  emd_26916_additional_1.map.gz emd_26916_additional_1.map.gz emd_26916_additional_2.map.gz emd_26916_additional_2.map.gz emd_26916_additional_3.map.gz emd_26916_additional_3.map.gz emd_26916_additional_4.map.gz emd_26916_additional_4.map.gz emd_26916_half_map_1.map.gz emd_26916_half_map_1.map.gz emd_26916_half_map_2.map.gz emd_26916_half_map_2.map.gz | 322.5 MB 322.5 MB 607.3 KB 328 MB 64.8 MB 64.8 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-26916 http://ftp.pdbj.org/pub/emdb/structures/EMD-26916 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-26916 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-26916 | HTTPS FTP |
-Related structure data
| Related structure data |  7uzqMC  7uz3C  7uzeC  7uzsC  7uzuC  7uzvC  7v07C  7v0kC  7v0mC  7v0qC  7v0sC  7v0tC  7v0uC  7v0xC  7v0yC  7v19C  8crqC  8crrC  8crtC  8cs9C  8cslC  8csvC  8cswC  8csxC  8csyC  8ct2C  8ct3C  8cteC C: citing same article ( M: atomic model generated by this map |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_26916.map.gz / Format: CCP4 / Size: 71.8 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_26916.map.gz / Format: CCP4 / Size: 71.8 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Main map used for model building/refinement. Density modified and cropped using phenix.resolve_cryo_em, resampled on fine grid using relion_image_handler. | ||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.415 Å | ||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Additional map: Half map 1 (unmodified)
| File | emd_26916_additional_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Half map 1 (unmodified) | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Additional map: Half map 2 (unmodified)
| File | emd_26916_additional_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Half map 2 (unmodified) | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Additional map: Mask used for FSC calculation.
| File | emd_26916_additional_3.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Mask used for FSC calculation. | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Additional map: Sharpened original map (B=52.5)
| File | emd_26916_additional_4.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Sharpened original map (B=52.5) | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: Half map 1 (cropped and resampled).
| File | emd_26916_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Half map 1 (cropped and resampled). | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: Half map 2 (cropped and resampled).
| File | emd_26916_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Half map 2 (cropped and resampled). | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : Ankyrin complex mixture purified from digitonin-solubilized eryth...
| Entire | Name: Ankyrin complex mixture purified from digitonin-solubilized erythrocyte ghost membranes |
|---|---|
| Components |
|
-Supramolecule #1: Ankyrin complex mixture purified from digitonin-solubilized eryth...
| Supramolecule | Name: Ankyrin complex mixture purified from digitonin-solubilized erythrocyte ghost membranes type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1-#3 Details: Particle set isolated by 3D classification from mixture mostly containing ankyrin complexes |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) / Organ: Blood / Tissue: Erythrocytes / Location in cell: Plasma membrane Homo sapiens (human) / Organ: Blood / Tissue: Erythrocytes / Location in cell: Plasma membrane |
-Macromolecule #1: Blood group Rh(CE) polypeptide
| Macromolecule | Name: Blood group Rh(CE) polypeptide / type: protein_or_peptide / ID: 1 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) / Organ: Blood / Tissue: Erythrocytes Homo sapiens (human) / Organ: Blood / Tissue: Erythrocytes |
| Molecular weight | Theoretical: 45.598918 KDa |
| Sequence | String: MSSKYPRSVR RCLPLWALTL EAALILLFYF FTHYDASLED QKGLVASYQV GQDLTVMAAL GLGFLTSNFR RHSWSSVAFN LFMLALGVQ WAILLDGFLS QFPPGKVVIT LFSIRLATMS AMSVLISAGA VLGKVNLAQL VVMVLVEVTA LGTLRMVISN I FNTDYHMN ...String: MSSKYPRSVR RCLPLWALTL EAALILLFYF FTHYDASLED QKGLVASYQV GQDLTVMAAL GLGFLTSNFR RHSWSSVAFN LFMLALGVQ WAILLDGFLS QFPPGKVVIT LFSIRLATMS AMSVLISAGA VLGKVNLAQL VVMVLVEVTA LGTLRMVISN I FNTDYHMN LRHFYVFAAY FGLTVAWCLP KPLPKGTEDN DQRATIPSLS AMLGALFLWM FWPSVNSPLL RSPIQRKNAM FN TYYALAV SVVTAISGSS LAHPQRKISM TYVHSAVLAG GVAVGTSCHL IPSPWLAMVL GLVAGLISIG GAKCLPVCCN RVL GIHHIS VMHSIFSLLG LLGEITYIVL LVLHTVWNGN GMIGFQVLLS IGELSLAIVI ALTSGLLTGL LLNLKIWKAP HVAK YFDDQ VFWKFPHLAV GF UniProtKB: Blood group Rh(CE) polypeptide |
-Macromolecule #2: Ammonium transporter Rh type A
| Macromolecule | Name: Ammonium transporter Rh type A / type: protein_or_peptide / ID: 2 / Number of copies: 2 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) / Organ: Blood / Tissue: Erythrocytes Homo sapiens (human) / Organ: Blood / Tissue: Erythrocytes |
| Molecular weight | Theoretical: 44.229629 KDa |
| Sequence | String: MRFTFPLMAI VLEIAMIVLF GLFVEYETDQ TVLEQLNITK PTDMGIFFEL YPLFQDVHVM IFVGFGFLMT FLKKYGFSSV GINLLVAAL GLQWGTIVQG ILQSQGQKFN IGIKNMINAD FSAATVLISF GAVLGKTSPT QMLIMTILEI VFFAHNEYLV S EIFKASDI ...String: MRFTFPLMAI VLEIAMIVLF GLFVEYETDQ TVLEQLNITK PTDMGIFFEL YPLFQDVHVM IFVGFGFLMT FLKKYGFSSV GINLLVAAL GLQWGTIVQG ILQSQGQKFN IGIKNMINAD FSAATVLISF GAVLGKTSPT QMLIMTILEI VFFAHNEYLV S EIFKASDI GASMTIHAFG AYFGLAVAGI LYRSGLRKGH ENEESAYYSD LFAMIGTLFL WMFWPSFNSA IAEPGDKQCR AI VNTYFSL AACVLTAFAF SSLVEHRGKL NMVHIQNATL AGGVAVGTCA DMAIHPFGSM IIGSIAGMVS VLGYKFLTPL FTT KLRIHD TCGVHNLHGL PGVVGGLAGI VAVAMGASNT SMAMQAAALG SSIGTAVVGG LMTGLILKLP LWGQPSDQNC YDDS VYWKV PKTR UniProtKB: Ammonium transporter Rh type A |
-Macromolecule #3: Ankyrin-1
| Macromolecule | Name: Ankyrin-1 / type: protein_or_peptide / ID: 3 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) / Organ: Blood / Tissue: Erythrocytes Homo sapiens (human) / Organ: Blood / Tissue: Erythrocytes |
| Molecular weight | Theoretical: 21.760494 KDa |
| Sequence | String: MPYSVGFREA DAATSFLRAA RSGNLDKALD HLRNGVDINT CNQNGLNGLH LASKEGHVKM VVELLHKEII LETTTKKGNT ALHIAALAG QDEVVRELVN YGANVNAQSQ KGFTPLYMAA QENHLEVVKF LLENGANQNV ATEDGFTPLA VALQQGHENV V AHLINYGT ...String: MPYSVGFREA DAATSFLRAA RSGNLDKALD HLRNGVDINT CNQNGLNGLH LASKEGHVKM VVELLHKEII LETTTKKGNT ALHIAALAG QDEVVRELVN YGANVNAQSQ KGFTPLYMAA QENHLEVVKF LLENGANQNV ATEDGFTPLA VALQQGHENV V AHLINYGT KGKVRLPALH IAARNDDTRT AAVLLQNDPN PDV UniProtKB: Ankyrin-1 |
-Macromolecule #4: CHOLESTEROL
| Macromolecule | Name: CHOLESTEROL / type: ligand / ID: 4 / Number of copies: 2 / Formula: CLR |
|---|---|
| Molecular weight | Theoretical: 386.654 Da |
| Chemical component information |  ChemComp-CLR: |
-Macromolecule #5: Digitonin
| Macromolecule | Name: Digitonin / type: ligand / ID: 5 / Number of copies: 2 / Formula: AJP |
|---|---|
| Molecular weight | Theoretical: 1.229312 KDa |
| Chemical component information |  ChemComp-AJP: |
-Macromolecule #6: water
| Macromolecule | Name: water / type: ligand / ID: 6 / Number of copies: 235 / Formula: HOH |
|---|---|
| Molecular weight | Theoretical: 18.015 Da |
| Chemical component information |  ChemComp-HOH: |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 8 mg/mL |
|---|---|
| Buffer | pH: 7.4 Details: Final gel filtration buffer contained 0.05% w/v digitonin, 130 mM KCl, 20 mM HEPES, pH 7.4, 1 mM ATP, 1 mM MgCl2, 1 mM PMSF. Peak fractions were concentrated to 8 mg/mL and 0.01% ( w/v ...Details: Final gel filtration buffer contained 0.05% w/v digitonin, 130 mM KCl, 20 mM HEPES, pH 7.4, 1 mM ATP, 1 mM MgCl2, 1 mM PMSF. Peak fractions were concentrated to 8 mg/mL and 0.01% ( w/v glycyrrhizic acid was added immediately prior to vitrification. |
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 277 K / Instrument: FEI VITROBOT MARK IV / Details: 4-6 seconds, wait time 30 seconds. |
| Details | Ankyrin complex mixture purified from digitonin-solubilized erythrocyte ghost membranes |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Specialist optics | Energy filter - Name: GIF Bioquantum / Energy filter - Slit width: 20 eV |
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Number grids imaged: 2 / Number real images: 14464 / Average exposure time: 2.5 sec. / Average electron dose: 58.0 e/Å2 / Details: Two grids were imaged in a single session. |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm / Nominal defocus max: 1.5 µm / Nominal defocus min: 0.5 µm |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller










































 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)