[English] 日本語
 Yorodumi
Yorodumi- EMDB-26625: Structure of Importin-4 bound to the H3-H4-ASF1 histone-histone c... -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | Structure of Importin-4 bound to the H3-H4-ASF1 histone-histone chaperone complex | |||||||||||||||
 Map data Map data | ||||||||||||||||
 Sample Sample |
| |||||||||||||||
 Keywords Keywords | Importin / Nuclear Import / Chaperone / Histones / H3 / H4 / ASF1 / NUCLEAR PROTEIN | |||||||||||||||
| Function / homology |  Function and homology information Function and homology informationDNA replication-dependent chromatin assembly / nuclear localization sequence binding / nucleosome disassembly / nuclear import signal receptor activity / protein localization to nucleus / small GTPase binding / protein import into nucleus / structural constituent of chromatin / nucleosome / nucleosome assembly ...DNA replication-dependent chromatin assembly / nuclear localization sequence binding / nucleosome disassembly / nuclear import signal receptor activity / protein localization to nucleus / small GTPase binding / protein import into nucleus / structural constituent of chromatin / nucleosome / nucleosome assembly / histone binding / protein heterodimerization activity / chromatin / protein-containing complex / DNA binding / nucleus / membrane / cytoplasm Similarity search - Function | |||||||||||||||
| Biological species |  Homo sapiens (human) / Homo sapiens (human) /  | |||||||||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 3.45 Å | |||||||||||||||
 Authors Authors | Bernardes NE / Chook YM / Fung HYJ / Chen Z / Li Y | |||||||||||||||
| Funding support |  United States, 4 items United States, 4 items
| |||||||||||||||
 Citation Citation |  Journal: Proc Natl Acad Sci U S A / Year: 2022 Journal: Proc Natl Acad Sci U S A / Year: 2022Title: Structure of IMPORTIN-4 bound to the H3-H4-ASF1 histone-histone chaperone complex. Authors: Natália Elisa Bernardes / Ho Yee Joyce Fung / Yang Li / Zhe Chen / Yuh Min Chook /  Abstract: IMPORTIN-4, the primary nuclear import receptor of core histones H3 and H4, binds the H3-H4 dimer and histone chaperone ASF1 prior to nuclear import. However, how H3-H3-ASF1 is recognized for ...IMPORTIN-4, the primary nuclear import receptor of core histones H3 and H4, binds the H3-H4 dimer and histone chaperone ASF1 prior to nuclear import. However, how H3-H3-ASF1 is recognized for transport cannot be explained by available crystal structures of IMPORTIN-4-histone tail peptide complexes. Our 3.5-Å IMPORTIN-4-H3-H4-ASF1 cryoelectron microscopy structure reveals the full nuclear import complex and shows a binding mode different from suggested by previous structures. The N-terminal half of IMPORTIN-4 clamps the globular H3-H4 domain and H3 αN helix, while its C-terminal half binds the H3 N-terminal tail weakly; tail contribution to binding energy is negligible. ASF1 binds H3-H4 without contacting IMPORTIN-4. Together, ASF1 and IMPORTIN-4 shield nucleosomal H3-H4 surfaces to chaperone and import it into the nucleus where RanGTP binds IMPORTIN-4, causing large conformational changes to release H3-H4-ASF1. This work explains how full-length H3-H4 binds IMPORTIN-4 in the cytoplasm and how it is released in the nucleus. | |||||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_26625.map.gz emd_26625.map.gz | 52.9 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-26625-v30.xml emd-26625-v30.xml emd-26625.xml emd-26625.xml | 22.4 KB 22.4 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_26625.png emd_26625.png | 42 KB | ||
| Filedesc metadata |  emd-26625.cif.gz emd-26625.cif.gz | 7.3 KB | ||
| Others |  emd_26625_half_map_1.map.gz emd_26625_half_map_1.map.gz emd_26625_half_map_2.map.gz emd_26625_half_map_2.map.gz | 95.6 MB 95.6 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-26625 http://ftp.pdbj.org/pub/emdb/structures/EMD-26625 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-26625 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-26625 | HTTPS FTP |
-Validation report
| Summary document |  emd_26625_validation.pdf.gz emd_26625_validation.pdf.gz | 972.5 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_26625_full_validation.pdf.gz emd_26625_full_validation.pdf.gz | 972 KB | Display | |
| Data in XML |  emd_26625_validation.xml.gz emd_26625_validation.xml.gz | 13.2 KB | Display | |
| Data in CIF |  emd_26625_validation.cif.gz emd_26625_validation.cif.gz | 15.2 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-26625 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-26625 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-26625 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-26625 | HTTPS FTP |
-Related structure data
| Related structure data |  7unkMC  8dyoC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_26625.map.gz / Format: CCP4 / Size: 103 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_26625.map.gz / Format: CCP4 / Size: 103 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.834 Å | ||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Half map: #1
| File | emd_26625_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
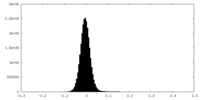
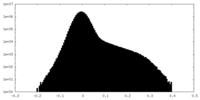
| Density Histograms |
-Half map: #2
| File | emd_26625_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
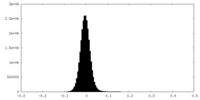
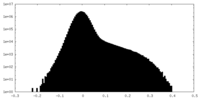
| Density Histograms |
- Sample components
Sample components
-Entire : Nuclear import complex of Imp4-H3-H4-Asf1
| Entire | Name: Nuclear import complex of Imp4-H3-H4-Asf1 |
|---|---|
| Components |
|
-Supramolecule #1: Nuclear import complex of Imp4-H3-H4-Asf1
| Supramolecule | Name: Nuclear import complex of Imp4-H3-H4-Asf1 / type: complex / ID: 1 / Parent: 0 / Macromolecule list: all |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 167 KDa |
-Macromolecule #1: Importin-4
| Macromolecule | Name: Importin-4 / type: protein_or_peptide / ID: 1 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 118.83207 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MESAGLEQLL RELLLPDTER IRRATEQLQI VLRAPAALPA LCDLLASAAD PQIRQFAAVL TRRRLNTRWR RLAAEQRESL KSLILTALQ RETEHCVSLS LAQLSATIFR KEGLEAWPQL LQLLQHSTHS PHSPEREMGL LLLSVVVTSR PEAFQPHHRE L LRLLNETL ...String: MESAGLEQLL RELLLPDTER IRRATEQLQI VLRAPAALPA LCDLLASAAD PQIRQFAAVL TRRRLNTRWR RLAAEQRESL KSLILTALQ RETEHCVSLS LAQLSATIFR KEGLEAWPQL LQLLQHSTHS PHSPEREMGL LLLSVVVTSR PEAFQPHHRE L LRLLNETL GEVGSPGLLF YSLRTLTTMA PYLSTEDVPL ARMLVPKLIM AMQTLIPIDE AKACEALEAL DELLESEVPV IT PYLSEVL TFCLEVARNV ALGNAIRIRI LCCLTFLVKV KSKALLKNRL LPPLLHTLFP IVAAEPPPGQ LDPEDQDSEE EEL EIELMG ETPKHFAVQV VDMLALHLPP EKLCPQLMPM LEEALRSESP YQRKAGLLVL AVLSDGAGDH IRQRLLPPLL QIVC KGLED PSQVVRNAAL FALGQFSENL QPHISSYSRE VMPLLLAYLK SVPLGHTHHL AKACYALENF VENLGPKVQP YLPEL MECM LQLLRNPSSP RAKELAVSAL GAIATAAQAS LLPYFPAIME HLREFLLTGR EDLQPVQIQS LETLGVLARA VGEPMR PLA EECCQLGLGL CDQVDDPDLR RCTYSLFAAL SGLMGEGLAP HLEQITTLML LSLRSTEGIV PQYDGSSSFL LFDDESD GE EEEELMDEDV EEEDDSEISG YSVENAFFDE KEDTCAAVGE ISVNTSVAFL PYMESVFEEV FKLLECPHLN VRKAAHEA L GQFCCALHKA CQSCPSEPNT AALQAALARV VPSYMQAVNR ERERQVVMAV LEALTGVLRS CGTLTLKPPG RLAELCGVL KAVLQRKTAC QDTDEEEEEE DDDQAEYDAM LLEHAGEAIP ALAAAAGGDS FAPFFAGFLP LLVCKTKQGC TVAEKSFAVG TLAETIQGL GAASAQFVSR LLPVLLSTAQ EADPEVRSNA IFGMGVLAEH GGHPAQEHFP KLLGLLFPLL ARERHDRVRD N ICGALARL LMASPTRKPE PQVLAALLHA LPLKEDLEEW VTIGRLFSFL YQSSPDQVID VAPELLRICS LILADNKIPP DT KAALLLL LTFLAKQHTD SFQAALGSLP VDKAQELQAV LGLS UniProtKB: Importin-4 |
-Macromolecule #2: Histone H3
| Macromolecule | Name: Histone H3 / type: protein_or_peptide / ID: 2 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism: |
| Molecular weight | Theoretical: 15.407075 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MARTKQTARK STGGKAPRKQ LATKAARKSA PATGGVKKPH RYRPGTVALR EIRRYQKSTE LLIRKLPFQR LVREIAQDFK TDLRFQSSA VMALQEASEA YLVGLFEDTN LCGIHAKRVT IMPKDIQLAR RIRGERA UniProtKB: Histone H3 |
-Macromolecule #3: Histone chaperone
| Macromolecule | Name: Histone chaperone / type: protein_or_peptide / ID: 3 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 31.62926 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MSIVSLLGIK VLNNPAKFTD PYEFEITFEC LESLKHDLEW KLTYVGSSRS LDHDQELDSI LVGPVPVGVN KFVFSADPPS AELIPASEL VSVTVILLSC SYDGREFVRV GYYVNNEYDE EELRENPPAK VQVDHIVRNI LAEKPRVTRF NIVWDNENEG D LYPPEQPG ...String: MSIVSLLGIK VLNNPAKFTD PYEFEITFEC LESLKHDLEW KLTYVGSSRS LDHDQELDSI LVGPVPVGVN KFVFSADPPS AELIPASEL VSVTVILLSC SYDGREFVRV GYYVNNEYDE EELRENPPAK VQVDHIVRNI LAEKPRVTRF NIVWDNENEG D LYPPEQPG VDDEEEEDDE EEDDDEDDED DEDDDQEDGE GEAEEAAEEE EEEEEKTEDN ETNLEEEEED IENSDGDEEE GE EEVGSVD KNEDGNDKKR RKIEGGSTDI ESTPKDAARS TN UniProtKB: Histone chaperone |
-Macromolecule #4: Histone H4
| Macromolecule | Name: Histone H4 / type: protein_or_peptide / ID: 4 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism: |
| Molecular weight | Theoretical: 11.394426 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MSGRGKGGKG LGKGGAKRHR KVLRDNIQGI TKPAIRRLAR RGGVKRISGL IYEETRGVLK VFLENVIRDA VTYTEHAKRK TVTAMDVVY ALKRQGRTLY GFGG UniProtKB: Histone H4 |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.5 |
|---|---|
| Grid | Model: Quantifoil / Material: COPPER / Support film - Material: CARBON / Support film - topology: HOLEY / Pretreatment - Type: GLOW DISCHARGE |
| Vitrification | Cryogen name: ETHANE / Instrument: FEI VITROBOT MARK IV |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 BIOQUANTUM (6k x 4k) / Average electron dose: 52.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: -2.5 µm / Nominal defocus min: -1.0 µm |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
+ Image processing
Image processing
-Atomic model buiding 1
| Refinement | Space: REAL / Protocol: RIGID BODY FIT |
|---|---|
| Output model |  PDB-7unk: |
 Movie
Movie Controller
Controller








 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)