+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Structure of G6PD-WT dimer | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | apo protein / OXIDOREDUCTASE | |||||||||
| Function / homology |  Function and homology information Function and homology informationpentose biosynthetic process / ribose phosphate biosynthetic process / response to iron(III) ion / positive regulation of calcium ion transmembrane transport via high voltage-gated calcium channel / glucose-6-phosphate dehydrogenase (NADP+) / glucose-6-phosphate dehydrogenase activity / Pentose phosphate pathway / pentose-phosphate shunt, oxidative branch / negative regulation of cell growth involved in cardiac muscle cell development / NADPH regeneration ...pentose biosynthetic process / ribose phosphate biosynthetic process / response to iron(III) ion / positive regulation of calcium ion transmembrane transport via high voltage-gated calcium channel / glucose-6-phosphate dehydrogenase (NADP+) / glucose-6-phosphate dehydrogenase activity / Pentose phosphate pathway / pentose-phosphate shunt, oxidative branch / negative regulation of cell growth involved in cardiac muscle cell development / NADPH regeneration / glucose 6-phosphate metabolic process / NADP+ metabolic process / pentose-phosphate shunt / D-glucose binding / NFE2L2 regulates pentose phosphate pathway genes / response to food / erythrocyte maturation / cholesterol biosynthetic process / negative regulation of reactive oxygen species metabolic process / regulation of neuron apoptotic process / glutathione metabolic process / substantia nigra development / TP53 Regulates Metabolic Genes / lipid metabolic process / cytoplasmic side of plasma membrane / centriolar satellite / glucose metabolic process / NADP binding / cellular response to oxidative stress / response to ethanol / intracellular membrane-bounded organelle / protein homodimerization activity / extracellular exosome / identical protein binding / membrane / cytosol / cytoplasm Similarity search - Function | |||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 3.5 Å | |||||||||
 Authors Authors | Wei X / Marmorstein R | |||||||||
| Funding support |  United States, 1 items United States, 1 items
| |||||||||
 Citation Citation |  Journal: To Be Published Journal: To Be PublishedTitle: Structure of G6PD-WT dimer Authors: Wei X / Marmorstein R | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_26425.map.gz emd_26425.map.gz | 20.7 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-26425-v30.xml emd-26425-v30.xml emd-26425.xml emd-26425.xml | 15.3 KB 15.3 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_26425.png emd_26425.png | 59.2 KB | ||
| Filedesc metadata |  emd-26425.cif.gz emd-26425.cif.gz | 5.6 KB | ||
| Others |  emd_26425_half_map_1.map.gz emd_26425_half_map_1.map.gz emd_26425_half_map_2.map.gz emd_26425_half_map_2.map.gz | 20.6 MB 20.6 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-26425 http://ftp.pdbj.org/pub/emdb/structures/EMD-26425 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-26425 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-26425 | HTTPS FTP |
-Validation report
| Summary document |  emd_26425_validation.pdf.gz emd_26425_validation.pdf.gz | 815.6 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_26425_full_validation.pdf.gz emd_26425_full_validation.pdf.gz | 815.2 KB | Display | |
| Data in XML |  emd_26425_validation.xml.gz emd_26425_validation.xml.gz | 10.3 KB | Display | |
| Data in CIF |  emd_26425_validation.cif.gz emd_26425_validation.cif.gz | 11.9 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-26425 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-26425 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-26425 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-26425 | HTTPS FTP |
-Related structure data
| Related structure data |  7uagMC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_26425.map.gz / Format: CCP4 / Size: 22.2 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_26425.map.gz / Format: CCP4 / Size: 22.2 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.83 Å | ||||||||||||||||||||||||||||||||||||
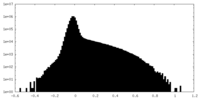
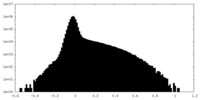
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Half map: #2
| File | emd_26425_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
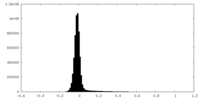
| Density Histograms |
-Half map: #1
| File | emd_26425_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
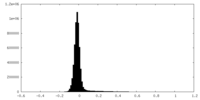
| Density Histograms |
- Sample components
Sample components
-Entire : G6PD protein
| Entire | Name: G6PD protein |
|---|---|
| Components |
|
-Supramolecule #1: G6PD protein
| Supramolecule | Name: G6PD protein / type: complex / ID: 1 / Parent: 0 / Macromolecule list: all |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 110 KDa |
-Macromolecule #1: Glucose-6-phosphate 1-dehydrogenase
| Macromolecule | Name: Glucose-6-phosphate 1-dehydrogenase / type: protein_or_peptide / ID: 1 / Number of copies: 2 / Enantiomer: LEVO / EC number: glucose-6-phosphate dehydrogenase (NADP+) |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 60.403754 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MAEQVALSRT QVCGILREEL FQGDAFHQSD THIFIIMGAS GDLAKKKIYP TIWWLFRDGL LPENTFIVGY ARSRLTVADI RKQSEPFFK ATPEEKLKLE DFFARNSYVA GQYDDAASYQ RLNSHMNALH LGSQANRLFY LALPPTVYEA VTKNIHESCM S QIGWNRII ...String: MAEQVALSRT QVCGILREEL FQGDAFHQSD THIFIIMGAS GDLAKKKIYP TIWWLFRDGL LPENTFIVGY ARSRLTVADI RKQSEPFFK ATPEEKLKLE DFFARNSYVA GQYDDAASYQ RLNSHMNALH LGSQANRLFY LALPPTVYEA VTKNIHESCM S QIGWNRII VEKPFGRDLQ SSDRLSNHIS SLFREDQIYR IDHYLGKEMV QNLMVLRFAN RIFGPIWNRD NIACVILTFK EP FGTEGRG GYFDEFGIIR DVMQNHLLQM LCLVAMEKPA STNSDDVRDE KVKVLKCISE VQANNVVLGQ YVGNPDGEGE ATK GYLDDP TVPRGSTTAT FAAVVLYVEN ERWDGVPFIL RCGKALNERK AEVRLQFHDV AGDIFHQQCK RNELVIRVQP NEAV YTKMM TKKPGMFFNP EESELDLTYG NRYKNVKLPD AYERLILDVF CGSQMHFVRS DELREAWRIF TPLLHQIELE KPKPI PYIY GSRGPTEADE LMKRVGFQYE GTYKWVNPHK LLEHHHHHH UniProtKB: Glucose-6-phosphate 1-dehydrogenase |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.5 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Average electron dose: 40.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: 2.0 µm / Nominal defocus min: 1.0 µm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller




 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)