+ データを開く
データを開く
- 基本情報
基本情報
| 登録情報 |  | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| タイトル | Structure of porcine kidney V-ATPase with SidK, Rotary State 1 | |||||||||||||||
 マップデータ マップデータ | Map used for model building. | |||||||||||||||
 試料 試料 |
| |||||||||||||||
 キーワード キーワード | proton translocation / complex / MEMBRANE PROTEIN | |||||||||||||||
| 機能・相同性 |  機能・相同性情報 機能・相同性情報ROS and RNS production in phagocytes / RHOA GTPase cycle / Transferrin endocytosis and recycling / Amino acids regulate mTORC1 / Ion channel transport / Insulin receptor recycling / symbiont-mediated suppression of host phagosome acidification / plasma membrane proton-transporting V-type ATPase complex / eye pigmentation / central nervous system maturation ...ROS and RNS production in phagocytes / RHOA GTPase cycle / Transferrin endocytosis and recycling / Amino acids regulate mTORC1 / Ion channel transport / Insulin receptor recycling / symbiont-mediated suppression of host phagosome acidification / plasma membrane proton-transporting V-type ATPase complex / eye pigmentation / central nervous system maturation / rostrocaudal neural tube patterning / proton-transporting V-type ATPase, V1 domain / positive regulation of transforming growth factor beta1 production / proton-transporting two-sector ATPase complex, catalytic domain / synaptic vesicle lumen acidification / proton-transporting V-type ATPase, V0 domain / cellular response to increased oxygen levels / vacuolar transport / lysosomal lumen acidification / vacuolar proton-transporting V-type ATPase, V1 domain / clathrin-coated vesicle membrane / endosome to plasma membrane protein transport / vacuolar proton-transporting V-type ATPase, V0 domain / proton-transporting V-type ATPase complex / vacuolar proton-transporting V-type ATPase complex / head morphogenesis / osteoclast development / vacuolar acidification / regulation of cellular pH / dendritic spine membrane / vacuolar membrane / microvillus / ATPase activator activity / regulation of MAPK cascade / autophagosome membrane / proton-transporting ATPase activity, rotational mechanism / positive regulation of Wnt signaling pathway / transporter activator activity / ATP metabolic process / H+-transporting two-sector ATPase / transport vesicle / angiotensin maturation / RNA endonuclease activity / proton transmembrane transport / small GTPase binding / transmembrane transport / synaptic vesicle membrane / positive regulation of canonical Wnt signaling pathway / melanosome / presynapse / signaling receptor activity / ATPase binding / intracellular iron ion homeostasis / early endosome / endosome membrane / lysosome / endosome / apical plasma membrane / lysosomal membrane / external side of plasma membrane / endoplasmic reticulum membrane / ATP hydrolysis activity / ATP binding / membrane / plasma membrane / cytosol / cytoplasm 類似検索 - 分子機能 | |||||||||||||||
| 生物種 |   | |||||||||||||||
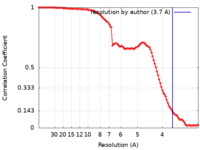
| 手法 | 単粒子再構成法 / クライオ電子顕微鏡法 / 解像度: 3.7 Å | |||||||||||||||
 データ登録者 データ登録者 | Tan YZ | |||||||||||||||
| 資金援助 |  カナダ, カナダ,  シンガポール, 4件 シンガポール, 4件
| |||||||||||||||
 引用 引用 |  ジャーナル: Life Sci Alliance / 年: 2022 ジャーナル: Life Sci Alliance / 年: 2022タイトル: CryoEM of endogenous mammalian V-ATPase interacting with the TLDc protein mEAK-7. 著者: Yong Zi Tan / Yazan M Abbas / Jing Ze Wu / Di Wu / Kristine A Keon / Geoffrey G Hesketh / Stephanie A Bueler / Anne-Claude Gingras / Carol V Robinson / Sergio Grinstein / John L Rubinstein /   要旨: V-ATPases are rotary proton pumps that serve as signaling hubs with numerous protein binding partners. CryoEM with exhaustive focused classification allowed detection of endogenous proteins ...V-ATPases are rotary proton pumps that serve as signaling hubs with numerous protein binding partners. CryoEM with exhaustive focused classification allowed detection of endogenous proteins associated with porcine kidney V-ATPase. An extra C subunit was found in ∼3% of complexes, whereas ∼1.6% of complexes bound mEAK-7, a protein with proposed roles in dauer formation in nematodes and mTOR signaling in mammals. High-resolution cryoEM of porcine kidney V-ATPase with recombinant mEAK-7 showed that mEAK-7's TLDc domain interacts with V-ATPase's stator, whereas its C-terminal α helix binds V-ATPase's rotor. This crosslink would be expected to inhibit rotary catalysis. However, unlike the yeast TLDc protein Oxr1p, exogenous mEAK-7 does not inhibit V-ATPase and mEAK-7 overexpression in cells does not alter lysosomal or phagosomal pH. Instead, cryoEM suggests that the mEAK-7:V-ATPase interaction is disrupted by ATP-induced rotation of the rotor. Comparison of Oxr1p and mEAK-7 binding explains this difference. These results show that V-ATPase binding by TLDc domain proteins can lead to effects ranging from strong inhibition to formation of labile interactions that are sensitive to the enzyme's activity. | |||||||||||||||
| 履歴 |
|
- 構造の表示
構造の表示
| 添付画像 |
|---|
- ダウンロードとリンク
ダウンロードとリンク
-EMDBアーカイブ
| マップデータ |  emd_26386.map.gz emd_26386.map.gz | 12.1 MB |  EMDBマップデータ形式 EMDBマップデータ形式 | |
|---|---|---|---|---|
| ヘッダ (付随情報) |  emd-26386-v30.xml emd-26386-v30.xml emd-26386.xml emd-26386.xml | 35.6 KB 35.6 KB | 表示 表示 |  EMDBヘッダ EMDBヘッダ |
| FSC (解像度算出) |  emd_26386_fsc.xml emd_26386_fsc.xml | 10.4 KB | 表示 |  FSCデータファイル FSCデータファイル |
| 画像 |  emd_26386.png emd_26386.png | 53.6 KB | ||
| Filedesc metadata |  emd-26386.cif.gz emd-26386.cif.gz | 9.9 KB | ||
| その他 |  emd_26386_additional_1.map.gz emd_26386_additional_1.map.gz emd_26386_half_map_1.map.gz emd_26386_half_map_1.map.gz emd_26386_half_map_2.map.gz emd_26386_half_map_2.map.gz | 97.4 MB 95.7 MB 95.7 MB | ||
| アーカイブディレクトリ |  http://ftp.pdbj.org/pub/emdb/structures/EMD-26386 http://ftp.pdbj.org/pub/emdb/structures/EMD-26386 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-26386 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-26386 | HTTPS FTP |
-検証レポート
| 文書・要旨 |  emd_26386_validation.pdf.gz emd_26386_validation.pdf.gz | 1 MB | 表示 |  EMDB検証レポート EMDB検証レポート |
|---|---|---|---|---|
| 文書・詳細版 |  emd_26386_full_validation.pdf.gz emd_26386_full_validation.pdf.gz | 1 MB | 表示 | |
| XML形式データ |  emd_26386_validation.xml.gz emd_26386_validation.xml.gz | 17 KB | 表示 | |
| CIF形式データ |  emd_26386_validation.cif.gz emd_26386_validation.cif.gz | 21.3 KB | 表示 | |
| アーカイブディレクトリ |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-26386 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-26386 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-26386 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-26386 | HTTPS FTP |
-関連構造データ
| 関連構造データ |  7u8pMC  7u8oC  7u8qC  7u8rC M: このマップから作成された原子モデル C: 同じ文献を引用 ( |
|---|---|
| 類似構造データ | 類似検索 - 機能・相同性  F&H 検索 F&H 検索 |
| 電子顕微鏡画像生データ |  EMPIAR-10874 (タイトル: Single-Particle CryoEM of mammalian V-ATPase with the TLDc domain protein mEAK7 bound (Various Datasets) EMPIAR-10874 (タイトル: Single-Particle CryoEM of mammalian V-ATPase with the TLDc domain protein mEAK7 bound (Various Datasets)Data size: 12.7 TB Data #1: Unaligned multiframe movies of Pig Kidney V-ATPase bound to mEAK-7 collected using Tundra [micrographs - multiframe] Data #2: Aligned and dose-weighted micrographs of Pig Kidney V-ATPase bound to mEAK-7 collected using Tundra [micrographs - single frame] Data #3: Polished particles of Pig Kidney V-ATPase bound to mEAK-7 collected using Tundra [picked particles - single frame - processed] Data #4: Unaligned multiframe movies of Pig Kidney V-ATPase bound to mEAK-7 collected using Titan Krios and Falcon4 [micrographs - multiframe] Data #5: Aligned and dose-weighted micrographs of Pig Kidney V-ATPase bound to mEAK-7 collected using Titan Krios and Falcon4 [micrographs - single frame] Data #6: Polished particles of Pig Kidney V-ATPase bound to mEAK-7 collected using Titan Krios and Falcon4 [picked particles - multiframe - processed] Data #7: Unaligned multiframe movies of Pig Kidney V-ATPase bound to mEAK-7deltaCterm collected using Titan Krios and Falcon4 [micrographs - multiframe] Data #8: Aligned and dose-weighted micrographs of Pig Kidney V-ATPase bound to mEAK-7deltaCterm collected using Titan Krios and Falcon4 [micrographs - single frame] Data #9: Polished particles of Pig Kidney V-ATPase bound to mEAK-7deltaCterm collected using Titan Krios and Falcon4 [picked particles - single frame - processed] Data #10: Unaligned multiframe movies of Pig Kidney V-ATPase bound to mEAK-7 with ATP collected using Titan Krios and Falcon4 [micrographs - multiframe] Data #11: Aligned and dose-weighted micrographs of Pig Kidney V-ATPase bound to mEAK-7 with ATP collected using Titan Krios and Falcon4 [micrographs - single frame] Data #12: Polished particles of Pig Kidney V-ATPase bound to mEAK-7 with ATP collected using Titan Krios and Falcon4 [picked particles - single frame - processed] Data #13: Unaligned multiframe movies of Pig Kidney V-ATPase bound to mEAK-7 with EDTA/EGTA collected using Titan Krios and Falcon4 [micrographs - multiframe] Data #14: Aligned and dose-weighted micrographs of Pig Kidney V-ATPase bound to mEAK-7 with EDTA/EGTA collected using Titan Krios and Falcon4 [micrographs - single frame] Data #15: Polished particles of Pig Kidney V-ATPase bound to mEAK-7 with EDTA/EGTA collected using Titan Krios and Falcon4 [picked particles - single frame - processed] Data #16: Unaligned multiframe movies of Pig Kidney V-ATPase bound to mEAK-7 with Calcium collected using Glacios with Selectris X and Falcon 4 [micrographs - multiframe] Data #17: Aligned and dose-weighted micrographs of Pig Kidney V-ATPase bound to mEAK-7 with Calcium collected using Glacios with Selectris X and Falcon 4 [micrographs - single frame] Data #18: Polished particles of Pig Kidney V-ATPase bound to mEAK-7 with Calcium collected using Glacios with Selectris X and Falcon 4 [picked particles - single frame - processed]) |
- リンク
リンク
| EMDBのページ |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| 「今月の分子」の関連する項目 |
- マップ
マップ
| ファイル |  ダウンロード / ファイル: emd_26386.map.gz / 形式: CCP4 / 大きさ: 13.1 MB / タイプ: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) ダウンロード / ファイル: emd_26386.map.gz / 形式: CCP4 / 大きさ: 13.1 MB / タイプ: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 注釈 | Map used for model building. | ||||||||||||||||||||||||||||||||||||
| 投影像・断面図 | 画像のコントロール
画像は Spider により作成 これらの図は立方格子座標系で作成されたものです | ||||||||||||||||||||||||||||||||||||
| ボクセルのサイズ | X=Y=Z: 1.52801 Å | ||||||||||||||||||||||||||||||||||||
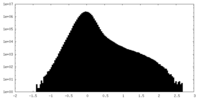
| 密度 |
| ||||||||||||||||||||||||||||||||||||
| 対称性 | 空間群: 1 | ||||||||||||||||||||||||||||||||||||
| 詳細 | EMDB XML:
|
-添付データ
-追加マップ: V-ATPase with SidK, Rotary State 1
| ファイル | emd_26386_additional_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 注釈 | V-ATPase with SidK, Rotary State 1 | ||||||||||||
| 投影像・断面図 |
| ||||||||||||
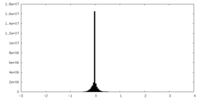
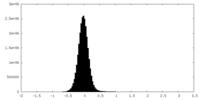
| 密度ヒストグラム |
-ハーフマップ: V-ATPase with SidK, Rotary State 1
| ファイル | emd_26386_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 注釈 | V-ATPase with SidK, Rotary State 1 | ||||||||||||
| 投影像・断面図 |
| ||||||||||||
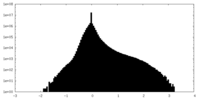
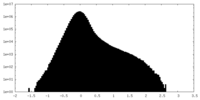
| 密度ヒストグラム |
-ハーフマップ: V-ATPase with SidK, Rotary State 1
| ファイル | emd_26386_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 注釈 | V-ATPase with SidK, Rotary State 1 | ||||||||||||
| 投影像・断面図 |
| ||||||||||||
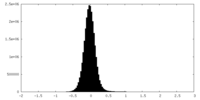
| 密度ヒストグラム |
- 試料の構成要素
試料の構成要素
+全体 : Porcine kidney V-ATPase with SidK, Rotary State 1
+超分子 #1: Porcine kidney V-ATPase with SidK, Rotary State 1
+分子 #1: V-type proton ATPase catalytic subunit A
+分子 #2: Vacuolar proton pump subunit B
+分子 #3: V-type proton ATPase subunit C
+分子 #4: V-type proton ATPase subunit D
+分子 #5: V-type proton ATPase subunit E 1
+分子 #6: V-type proton ATPase subunit F
+分子 #7: V-type proton ATPase subunit G
+分子 #8: Bacterial effector protein SidK
+分子 #9: V-type proton ATPase subunit H
+分子 #10: V-type proton ATPase subunit a
+分子 #11: V-type proton ATPase 21 kDa proteolipid subunit isoform 1
+分子 #12: ATPase H+ transporting accessory protein 1
+分子 #13: V-type proton ATPase subunit
+分子 #14: V-type proton ATPase subunit
+分子 #15: Ribonuclease kappa
+分子 #16: V-type proton ATPase proteolipid subunit
+分子 #17: ATPase H(+)-transporting lysosomal accessory protein 2
+分子 #18: ADENOSINE-5'-DIPHOSPHATE
-実験情報
-構造解析
| 手法 | クライオ電子顕微鏡法 |
|---|---|
 解析 解析 | 単粒子再構成法 |
| 試料の集合状態 | particle |
- 試料調製
試料調製
| 緩衝液 | pH: 7.4 |
|---|---|
| 凍結 | 凍結剤: ETHANE |
- 電子顕微鏡法
電子顕微鏡法
| 顕微鏡 | FEI TITAN KRIOS |
|---|---|
| 撮影 | フィルム・検出器のモデル: FEI FALCON IV (4k x 4k) 平均電子線量: 40.0 e/Å2 |
| 電子線 | 加速電圧: 300 kV / 電子線源:  FIELD EMISSION GUN FIELD EMISSION GUN |
| 電子光学系 | 照射モード: FLOOD BEAM / 撮影モード: BRIGHT FIELD / 最大 デフォーカス(公称値): 3.911445 µm / 最小 デフォーカス(公称値): 0.1 µm |
| 実験機器 |  モデル: Titan Krios / 画像提供: FEI Company |
 ムービー
ムービー コントローラー
コントローラー










 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)