[English] 日本語
 Yorodumi
Yorodumi- EMDB-26017: Complex GGGG of AMPA-subtype iGluR GluA2 in complex with auxiliar... -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | Complex GGGG of AMPA-subtype iGluR GluA2 in complex with auxiliary subunit gamma2 (Stargazin) at low glutamate concentration (20 uM) in the presence of cyclothiazide (100 uM) | |||||||||||||||
 Map data Map data | Complex GGGG of AMPA-subtype iGluR GluA2 in complex with auxiliary subunit gamma2 (Stargazin) at low glutamate concentration (20 uM) in the presence of cyclothiazide (100 uM) | |||||||||||||||
 Sample Sample |
| |||||||||||||||
 Keywords Keywords | AMPA / iGluR / Stargazin / cryo-EM / complex / agonist / positive allosteric modulator / subconductance level / opening / gating / glutamate / MEMBRANE PROTEIN | |||||||||||||||
| Function / homology |  Function and homology information Function and homology informationpostsynaptic neurotransmitter receptor diffusion trapping / regulation of AMPA receptor activity / Trafficking of AMPA receptors / channel regulator activity / LGI-ADAM interactions / spine synapse / dendritic spine neck / neurotransmitter receptor localization to postsynaptic specialization membrane / dendritic spine head / cellular response to amine stimulus ...postsynaptic neurotransmitter receptor diffusion trapping / regulation of AMPA receptor activity / Trafficking of AMPA receptors / channel regulator activity / LGI-ADAM interactions / spine synapse / dendritic spine neck / neurotransmitter receptor localization to postsynaptic specialization membrane / dendritic spine head / cellular response to amine stimulus / Activation of AMPA receptors / perisynaptic space / ligand-gated monoatomic cation channel activity / AMPA glutamate receptor activity / transmission of nerve impulse / Trafficking of GluR2-containing AMPA receptors / response to lithium ion / kainate selective glutamate receptor activity / AMPA glutamate receptor complex / cellular response to glycine / extracellularly glutamate-gated ion channel activity / ionotropic glutamate receptor complex / immunoglobulin binding / asymmetric synapse / conditioned place preference / excitatory synapse / regulation of receptor recycling / glutamate receptor binding / Unblocking of NMDA receptors, glutamate binding and activation / positive regulation of synaptic transmission / positive regulation of synaptic transmission, glutamatergic / protein targeting / voltage-gated calcium channel activity / regulation of synaptic transmission, glutamatergic / response to fungicide / glutamate-gated receptor activity / cytoskeletal protein binding / regulation of long-term synaptic depression / extracellular ligand-gated monoatomic ion channel activity / cellular response to brain-derived neurotrophic factor stimulus / glutamate-gated calcium ion channel activity / presynaptic active zone membrane / somatodendritic compartment / ionotropic glutamate receptor binding / dendrite membrane / ligand-gated monoatomic ion channel activity involved in regulation of presynaptic membrane potential / dendrite cytoplasm / ionotropic glutamate receptor signaling pathway / synaptic membrane / SNARE binding / dendritic shaft / transmitter-gated monoatomic ion channel activity involved in regulation of postsynaptic membrane potential / synaptic transmission, glutamatergic / PDZ domain binding / protein tetramerization / establishment of protein localization / postsynaptic density membrane / modulation of chemical synaptic transmission / cerebral cortex development / receptor internalization / Schaffer collateral - CA1 synapse / terminal bouton / synaptic vesicle / synaptic vesicle membrane / intracellular protein localization / presynapse / signaling receptor activity / amyloid-beta binding / growth cone / presynaptic membrane / scaffold protein binding / perikaryon / chemical synaptic transmission / dendritic spine / postsynaptic membrane / neuron projection / postsynaptic density / axon / external side of plasma membrane / neuronal cell body / synapse / dendrite / protein kinase binding / protein-containing complex binding / glutamatergic synapse / cell surface / endoplasmic reticulum / protein-containing complex / identical protein binding / membrane / plasma membrane Similarity search - Function | |||||||||||||||
| Biological species |  | |||||||||||||||
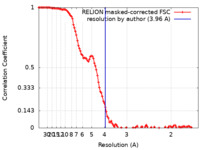
| Method | single particle reconstruction / cryo EM / Resolution: 3.96 Å | |||||||||||||||
 Authors Authors | Yelshanskaya MV / Sobolevsky AI | |||||||||||||||
| Funding support |  United States, 4 items United States, 4 items
| |||||||||||||||
 Citation Citation |  Journal: Nature / Year: 2022 Journal: Nature / Year: 2022Title: Opening of glutamate receptor channel to subconductance levels. Authors: Maria V Yelshanskaya / Dhilon S Patel / Christopher M Kottke / Maria G Kurnikova / Alexander I Sobolevsky /  Abstract: Ionotropic glutamate receptors (iGluRs) are tetrameric ligand-gated ion channels that open their pores in response to binding of the agonist glutamate. An ionic current through a single iGluR channel ...Ionotropic glutamate receptors (iGluRs) are tetrameric ligand-gated ion channels that open their pores in response to binding of the agonist glutamate. An ionic current through a single iGluR channel shows up to four discrete conductance levels (O1-O4). Higher conductance levels have been associated with an increased number of agonist molecules bound to four individual ligand-binding domains (LBDs). Here we determine structures of a synaptic complex of AMPA-subtype iGluR and the auxiliary subunit γ2 in non-desensitizing conditions with various occupancy of the LBDs by glutamate. We show that glutamate binds to LBDs of subunits B and D only after it is already bound to at least the same number of LBDs that belong to subunits A and C. Our structures combined with single-channel recordings, molecular dynamics simulations and machine-learning analysis suggest that channel opening requires agonist binding to at least two LBDs. Conversely, agonist binding to all four LBDs does not guarantee maximal channel conductance and favours subconductance states O1 and O2, with O3 and O4 being rare and not captured structurally. The lack of subunit independence and low efficiency coupling of glutamate binding to channel opening underlie the gating of synaptic complexes to submaximal conductance levels, which provide a potential for upregulation of synaptic activity. | |||||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_26017.map.gz emd_26017.map.gz | 11.7 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-26017-v30.xml emd-26017-v30.xml emd-26017.xml emd-26017.xml | 13.4 KB 13.4 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_26017_fsc.xml emd_26017_fsc.xml | 11.4 KB | Display |  FSC data file FSC data file |
| Images |  emd_26017.png emd_26017.png | 53.8 KB | ||
| Filedesc metadata |  emd-26017.cif.gz emd-26017.cif.gz | 6 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-26017 http://ftp.pdbj.org/pub/emdb/structures/EMD-26017 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-26017 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-26017 | HTTPS FTP |
-Validation report
| Summary document |  emd_26017_validation.pdf.gz emd_26017_validation.pdf.gz | 429.9 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_26017_full_validation.pdf.gz emd_26017_full_validation.pdf.gz | 429.4 KB | Display | |
| Data in XML |  emd_26017_validation.xml.gz emd_26017_validation.xml.gz | 12.2 KB | Display | |
| Data in CIF |  emd_26017_validation.cif.gz emd_26017_validation.cif.gz | 16.3 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-26017 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-26017 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-26017 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-26017 | HTTPS FTP |
-Related structure data
| Related structure data |  7tnpMC  7tnjC  7tnkC  7tnlC  7tnmC  7tnnC  7tnoC C: citing same article ( M: atomic model generated by this map |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_26017.map.gz / Format: CCP4 / Size: 125 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_26017.map.gz / Format: CCP4 / Size: 125 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Complex GGGG of AMPA-subtype iGluR GluA2 in complex with auxiliary subunit gamma2 (Stargazin) at low glutamate concentration (20 uM) in the presence of cyclothiazide (100 uM) | ||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.83 Å | ||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
- Sample components
Sample components
-Entire : Ionotropic glutamate receptor GluA2 in complex with auxiliary sub...
| Entire | Name: Ionotropic glutamate receptor GluA2 in complex with auxiliary subunit gamma2 or Stargazin |
|---|---|
| Components |
|
-Supramolecule #1: Ionotropic glutamate receptor GluA2 in complex with auxiliary sub...
| Supramolecule | Name: Ionotropic glutamate receptor GluA2 in complex with auxiliary subunit gamma2 or Stargazin type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1 |
|---|---|
| Source (natural) | Organism:  |
-Macromolecule #1: Isoform Flip of Glutamate receptor 2,Voltage-dependent calcium ch...
| Macromolecule | Name: Isoform Flip of Glutamate receptor 2,Voltage-dependent calcium channel gamma-3 subunit chimera type: protein_or_peptide / ID: 1 / Number of copies: 4 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 115.444922 KDa |
| Recombinant expression | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: NSIQIGGLFP RGADQEYSAF RVGMVQFSTS EFRLTPHIDN LEVANSFAVT NAFCSQFSRG VYAIFGFYDK KSVNTITSFC GTLHVSFIT PSFPTDGTHP FVIQMRPDLK GALLSLIEYY QWDKFAYLYD SDRGLSTLQA VLDSAAEKKW QVTAINVGNI N NDKKDETY ...String: NSIQIGGLFP RGADQEYSAF RVGMVQFSTS EFRLTPHIDN LEVANSFAVT NAFCSQFSRG VYAIFGFYDK KSVNTITSFC GTLHVSFIT PSFPTDGTHP FVIQMRPDLK GALLSLIEYY QWDKFAYLYD SDRGLSTLQA VLDSAAEKKW QVTAINVGNI N NDKKDETY RSLFQDLELK KERRVILDCE RDKVNDIVDQ VITIGKHVKG YHYIIANLGF TDGDLLKIQF GGAEVSGFQI VD YDDSLVS KFIERWSTLE EKEYPGAHTA TIKYTSALTY DAVQVMTEAF RNLRKQRIEI SRRGNAGDCL ANPAVPWGQG VEI ERALKQ VQVEGLSGNI KFDQNGKRIN YTINIMELKT NGPRKIGYWS EVDKMVLTED DTSGLEQKTV VVTTILESPY VMMK KNHEM LEGNERYEGY CVDLAAEIAK HCGFKYKLTI VGDGKYGARD ADTKIWNGMV GELVYGKADI AIAPLTITLV REEVI DFSK PFMSLGISIM IKKPQKSKPG VFSFLDPLAY EIWMCIVFAY IGVSVVLFLV SRFSPYEWHT EEFEDGRETQ SSESTN EFG IFNSLWFSLG AFMQQGCDIS PRSLSGRIVG GVWWFFTLII ISSYTANLAA FLTVERMVSP IESAEDLSKQ TEIAYGT LD SGSTKEFFRR SKIAVFDKMW TYMRSAEPSV FVRTTAEGVA RVRKSKGKYA YLLESTMNEY IEQRKPCDTM KVGGNLDS K GYGIATPKGS SLGTPVNLAV LKLSEQGVLD KLKNKWWYDK GECGAKDSGS KEKTSALSLS NVAGVFYILV GGLGLAMLV ALIEFCYKSR AEAKRMKGTG LFDRGVQMLL TTVGAFAAFS LMTIAVGTDY WLYSRGVCKT KSVSEDETSK KNEEVMTHSG LWRTCCLEG NFKGLCKQID HFPEDADYEA DTAEYFLRAV RASSIFPILS VILLFMGGLC IAASEFYKTR HNIILSAGIF F VSAGLSNI IGIIVYISAN AGDPSKSDSK KNSYSYGWSF YFGALSFIIA EMVGVLAVHM FIDRHKQLTG GLVPR UniProtKB: Glutamate receptor 2, Voltage-dependent calcium channel gamma-3 subunit |
-Macromolecule #2: GLUTAMIC ACID
| Macromolecule | Name: GLUTAMIC ACID / type: ligand / ID: 2 / Number of copies: 4 / Formula: GLU |
|---|---|
| Molecular weight | Theoretical: 147.129 Da |
| Chemical component information |  ChemComp-GLU: |
-Macromolecule #3: CYCLOTHIAZIDE
| Macromolecule | Name: CYCLOTHIAZIDE / type: ligand / ID: 3 / Number of copies: 4 / Formula: CYZ |
|---|---|
| Molecular weight | Theoretical: 389.878 Da |
| Chemical component information | 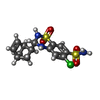 ChemComp-CYZ: |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 8 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Average electron dose: 58.5 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: 2.5 µm / Nominal defocus min: 1.0 µm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller















 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)