[English] 日本語
 Yorodumi
Yorodumi- EMDB-25687: CryoEM structure of the HCMV Pentamer gH/gL/UL128/UL130/UL131A in... -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | CryoEM structure of the HCMV Pentamer gH/gL/UL128/UL130/UL131A in complex with NRP2 and neutralizing fabs 8I21 and 13H11 | |||||||||
 Map data Map data | Composite map of combined focused refinement maps. Used for model refinements. | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | glycoprotein complex / antibody complex / Neuropilin 2 / VIRAL PROTEIN / VIRAL PROTEIN-Immune System complex | |||||||||
| Function / homology |  Function and homology information Function and homology informationvestibulocochlear nerve structural organization / dorsal root ganglion morphogenesis / ventral trunk neural crest cell migration / sympathetic neuron projection guidance / facioacoustic ganglion development / trigeminal ganglion development / trigeminal nerve structural organization / sensory neuron axon guidance / facial nerve structural organization / branchiomotor neuron axon guidance ...vestibulocochlear nerve structural organization / dorsal root ganglion morphogenesis / ventral trunk neural crest cell migration / sympathetic neuron projection guidance / facioacoustic ganglion development / trigeminal ganglion development / trigeminal nerve structural organization / sensory neuron axon guidance / facial nerve structural organization / branchiomotor neuron axon guidance / gonadotrophin-releasing hormone neuronal migration to the hypothalamus / axon extension involved in axon guidance / sympathetic neuron projection extension / NrCAM interactions / Neurophilin interactions with VEGF and VEGFR / neural crest cell migration involved in autonomic nervous system development / sympathetic ganglion development / vascular endothelial growth factor receptor activity / nerve development / semaphorin receptor complex / semaphorin receptor activity / outflow tract septum morphogenesis / regulation of postsynapse organization / negative chemotaxis / growth factor binding / cytokine binding / semaphorin-plexin signaling pathway / positive regulation of endothelial cell proliferation / positive regulation of endothelial cell migration / axon guidance / cellular response to leukemia inhibitory factor / signaling receptor activity / heparin binding / angiogenesis / host cell endosome / host cell Golgi apparatus / entry receptor-mediated virion attachment to host cell / postsynaptic membrane / cell adhesion / fusion of virus membrane with host plasma membrane / axon / viral envelope / symbiont entry into host cell / host cell plasma membrane / virion membrane / glutamatergic synapse / extracellular region / metal ion binding / identical protein binding / membrane / plasma membrane Similarity search - Function | |||||||||
| Biological species |   Human betaherpesvirus 5 / Human betaherpesvirus 5 /  Homo sapiens (human) Homo sapiens (human) | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 3.1 Å | |||||||||
 Authors Authors | Kschonsak M / Johnson MC | |||||||||
| Funding support | 1 items
| |||||||||
 Citation Citation |  Journal: Sci Adv / Year: 2022 Journal: Sci Adv / Year: 2022Title: Structural basis for HCMV Pentamer receptor recognition and antibody neutralization. Authors: Marc Kschonsak / Matthew C Johnson / Rachel Schelling / Evan M Green / Lionel Rougé / Hoangdung Ho / Nidhi Patel / Cem Kilic / Edward Kraft / Christopher P Arthur / Alexis L Rohou / ...Authors: Marc Kschonsak / Matthew C Johnson / Rachel Schelling / Evan M Green / Lionel Rougé / Hoangdung Ho / Nidhi Patel / Cem Kilic / Edward Kraft / Christopher P Arthur / Alexis L Rohou / Laetitia Comps-Agrar / Nadia Martinez-Martin / Laurent Perez / Jian Payandeh / Claudio Ciferri /   Abstract: Human cytomegalovirus (HCMV) represents the viral leading cause of congenital birth defects and uses the gH/gL/UL128-130-131A complex (Pentamer) to enter different cell types, including epithelial ...Human cytomegalovirus (HCMV) represents the viral leading cause of congenital birth defects and uses the gH/gL/UL128-130-131A complex (Pentamer) to enter different cell types, including epithelial and endothelial cells. Upon infection, Pentamer elicits the most potent neutralizing response against HCMV, representing a key vaccine candidate. Despite its relevance, the structural basis for Pentamer receptor recognition and antibody neutralization is largely unknown. Here, we determine the structures of Pentamer bound to neuropilin 2 (NRP2) and a set of potent neutralizing antibodies against HCMV. Moreover, we identify thrombomodulin (THBD) as a functional HCMV receptor and determine the structures of the Pentamer-THBD complex. Unexpectedly, both NRP2 and THBD also promote dimerization of Pentamer. Our results provide a framework for understanding HCMV receptor engagement, cell entry, antibody neutralization, and outline strategies for antiviral therapies against HCMV. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_25687.map.gz emd_25687.map.gz | 2.3 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-25687-v30.xml emd-25687-v30.xml emd-25687.xml emd-25687.xml | 55.1 KB 55.1 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_25687.png emd_25687.png | 45 KB | ||
| Filedesc metadata |  emd-25687.cif.gz emd-25687.cif.gz | 9.9 KB | ||
| Others |  emd_25687_additional_1.map.gz emd_25687_additional_1.map.gz emd_25687_additional_10.map.gz emd_25687_additional_10.map.gz emd_25687_additional_11.map.gz emd_25687_additional_11.map.gz emd_25687_additional_12.map.gz emd_25687_additional_12.map.gz emd_25687_additional_13.map.gz emd_25687_additional_13.map.gz emd_25687_additional_2.map.gz emd_25687_additional_2.map.gz emd_25687_additional_3.map.gz emd_25687_additional_3.map.gz emd_25687_additional_4.map.gz emd_25687_additional_4.map.gz emd_25687_additional_5.map.gz emd_25687_additional_5.map.gz emd_25687_additional_6.map.gz emd_25687_additional_6.map.gz emd_25687_additional_7.map.gz emd_25687_additional_7.map.gz emd_25687_additional_8.map.gz emd_25687_additional_8.map.gz emd_25687_additional_9.map.gz emd_25687_additional_9.map.gz | 77.5 MB 77.5 MB 22.5 MB 1.2 MB 840.1 KB 77.5 MB 22.5 MB 22.5 MB 22.5 MB 77.6 MB 22.5 MB 22.5 MB 2.2 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-25687 http://ftp.pdbj.org/pub/emdb/structures/EMD-25687 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-25687 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-25687 | HTTPS FTP |
-Related structure data
| Related structure data |  7t4sMC  7t4qC  7t4rC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_25687.map.gz / Format: CCP4 / Size: 87.4 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_25687.map.gz / Format: CCP4 / Size: 87.4 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Composite map of combined focused refinement maps. Used for model refinements. | ||||||||||||||||||||||||||||||||||||
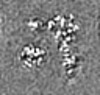
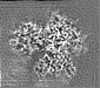
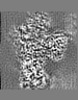
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.3514 Å | ||||||||||||||||||||||||||||||||||||
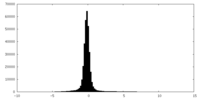
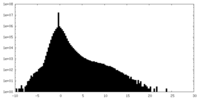
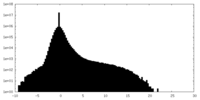
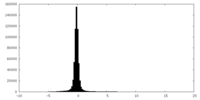
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
+Additional map: Non-sharpened, full map of overall (non-focused)refinement
+Additional map: Non-sharpened, full map of focused refinement on 8I21-UL128 region
+Additional map: Half map 1 of focused refinement on 8I21-UL128 region
+Additional map: Density modified map of focused refined 8I12-UL128 region....
+Additional map: Density modified map of focused refined NRP2-UL region....
+Additional map: Non-sharpened, full map of focused refinement on NRP2-UL region
+Additional map: Half map 1 of focused refinement on NRP2-UL region
+Additional map: Half map 2 of focused refinement on NRP2-UL region
+Additional map: Half map 2 of focused refinement on 8I21-UL128 region
+Additional map: Non-sharpened, full map of focused refinement on 13H11-gH region
+Additional map: Half map 1 of focused refinement on 13H11-gH region
+Additional map: Half map 2 of focused refinement on 13H11-gH region
+Additional map: Density modified map of focused refined 13H11-gH region....
- Sample components
Sample components
+Entire : Pentameric complex of HCMV proteins gH, gL, UL128, UL130, UL131A ...
+Supramolecule #1: Pentameric complex of HCMV proteins gH, gL, UL128, UL130, UL131A ...
+Macromolecule #1: Envelope glycoprotein H
+Macromolecule #2: Envelope glycoprotein L
+Macromolecule #3: Envelope protein UL128
+Macromolecule #4: Envelope glycoprotein UL130
+Macromolecule #5: Envelope protein UL131A
+Macromolecule #6: Neuropilin-2
+Macromolecule #7: Fab 8I21 heavy chain
+Macromolecule #8: Fab 13H11 heavy chain
+Macromolecule #9: Fab 13H11 light chain
+Macromolecule #10: Fab 8I21 light chain
+Macromolecule #11: 2-acetamido-2-deoxy-beta-D-glucopyranose
+Macromolecule #12: CALCIUM ION
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 1 mg/mL | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Buffer | pH: 7.5 Component:
| |||||||||
| Grid | Model: UltrAuFoil R0.6/1 / Material: GOLD / Mesh: 300 / Pretreatment - Type: PLASMA CLEANING / Pretreatment - Time: 20 sec. | |||||||||
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 277.15 K / Instrument: LEICA EM GP / Details: blot for 3.5 seconds before plunging. | |||||||||
| Details | Sample was mildly crosslinked with 0.025% glutaraldehyde, incubated for 10 min at room temperature and quenched with 9 mM TRIS pH 7.5 |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Detector mode: COUNTING / Number grids imaged: 4 / Number real images: 21357 / Average exposure time: 10.0 sec. / Average electron dose: 52.0 e/Å2 Details: Images were collected in movie-mode at 4 frames/second. |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm / Nominal defocus max: 1.5 µm / Nominal defocus min: 0.5 µm / Nominal magnification: 165000 |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller









 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)