+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-24940 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | 44SR3C ribosomal particle class 2 | |||||||||
 Map data Map data | Refine 3D map | |||||||||
 Sample Sample |
| |||||||||
| Biological species |  | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 3.3 Å | |||||||||
 Authors Authors | Ortega J / Seffouh A | |||||||||
| Funding support |  Canada, 1 items Canada, 1 items
| |||||||||
 Citation Citation |  Journal: Nucleic Acids Res / Year: 2022 Journal: Nucleic Acids Res / Year: 2022Title: RbgA ensures the correct timing in the maturation of the 50S subunits functional sites. Authors: Amal Seffouh / Chirstian Trahan / Tanzila Wasi / Nikhil Jain / Kaustuv Basu / Robert A Britton / Marlene Oeffinger / Joaquin Ortega /   Abstract: RbgA is an essential protein for the assembly of the 50S subunit in Bacillus subtilis. Depletion of RbgA leads to the accumulation of the 45S intermediate. A strain expressing a RbgA variant with ...RbgA is an essential protein for the assembly of the 50S subunit in Bacillus subtilis. Depletion of RbgA leads to the accumulation of the 45S intermediate. A strain expressing a RbgA variant with reduced GTPase activity generates spontaneous suppressor mutations in uL6. Each suppressor strain accumulates a unique 44S intermediate. We reasoned that characterizing the structure of these mutant 44S intermediates may explain why RbgA is required to catalyze the folding of the 50S functional sites. We found that in the 44S particles, rRNA helices H42 and H97, near the binding site of uL6, adopt a flexible conformation and allow the central protuberance and functional sites in the mutant 44S particles to mature in any order. Instead, the wild-type 45S particles exhibit a stable H42-H97 interaction and their functional sites always mature last. The dependence on RbgA was also less pronounced in the 44S particles. We concluded that the binding of uL6 pauses the maturation of the functional sites, but the central protuberance continues to fold. RbgA exclusively binds intermediates with a formed central protuberance and licenses the folding of the functional sites. Through this mechanism, RbgA ensures that the functional sites of the 50S mature last. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_24940.map.gz emd_24940.map.gz | 112.8 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-24940-v30.xml emd-24940-v30.xml emd-24940.xml emd-24940.xml | 9.6 KB 9.6 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_24940_fsc.xml emd_24940_fsc.xml | 12 KB | Display |  FSC data file FSC data file |
| Images |  emd_24940.png emd_24940.png | 64.7 KB | ||
| Others |  emd_24940_additional_1.map.gz emd_24940_additional_1.map.gz | 23.6 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-24940 http://ftp.pdbj.org/pub/emdb/structures/EMD-24940 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-24940 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-24940 | HTTPS FTP |
-Validation report
| Summary document |  emd_24940_validation.pdf.gz emd_24940_validation.pdf.gz | 515.8 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_24940_full_validation.pdf.gz emd_24940_full_validation.pdf.gz | 515.4 KB | Display | |
| Data in XML |  emd_24940_validation.xml.gz emd_24940_validation.xml.gz | 12.7 KB | Display | |
| Data in CIF |  emd_24940_validation.cif.gz emd_24940_validation.cif.gz | 16.9 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-24940 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-24940 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-24940 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-24940 | HTTPS FTP |
-Related structure data
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_24940.map.gz / Format: CCP4 / Size: 144.7 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_24940.map.gz / Format: CCP4 / Size: 144.7 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Refine 3D map | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.855 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
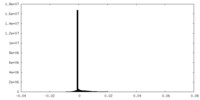
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
-Additional map: Postprocess map
| File | emd_24940_additional_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Postprocess map | ||||||||||||
| Projections & Slices |
| ||||||||||||
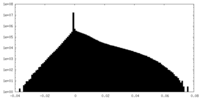
| Density Histograms |
- Sample components
Sample components
-Entire : 44S_R3C_ribosomal_particle class 2
| Entire | Name: 44S_R3C_ribosomal_particle class 2 |
|---|---|
| Components |
|
-Supramolecule #1: 44S_R3C_ribosomal_particle class 2
| Supramolecule | Name: 44S_R3C_ribosomal_particle class 2 / type: complex / ID: 1 / Parent: 0 |
|---|---|
| Source (natural) | Organism:  |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.5 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 BIOQUANTUM (6k x 4k) / Average electron dose: 50.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller



























 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)