+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-24208 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Structure of PPPA bound human ACAT2 | |||||||||
 Map data Map data | Structure of PPPA bound human ACAT2 | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | Inhibitor / MEMBRANE PROTEIN / Transferase-Inhibitor complex | |||||||||
| Function / homology |  Function and homology information Function and homology informationsterol O-acyltransferase / sterol O-acyltransferase activity / cholesterol O-acyltransferase activity / macrophage derived foam cell differentiation / cholesterol storage / very-low-density lipoprotein particle assembly / fatty-acyl-CoA binding / low-density lipoprotein particle clearance / intestinal cholesterol absorption / LDL clearance ...sterol O-acyltransferase / sterol O-acyltransferase activity / cholesterol O-acyltransferase activity / macrophage derived foam cell differentiation / cholesterol storage / very-low-density lipoprotein particle assembly / fatty-acyl-CoA binding / low-density lipoprotein particle clearance / intestinal cholesterol absorption / LDL clearance / cholesterol efflux / cholesterol binding / acyltransferase activity / brush border / cholesterol metabolic process / cholesterol homeostasis / endoplasmic reticulum membrane / endoplasmic reticulum Similarity search - Function | |||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 3.87 Å | |||||||||
 Authors Authors | Li X / Long T | |||||||||
| Funding support | 1 items
| |||||||||
 Citation Citation |  Journal: Structure / Year: 2021 Journal: Structure / Year: 2021Title: Molecular structures of human ACAT2 disclose mechanism for selective inhibition. Authors: Tao Long / Yang Liu / Xiaochun Li /  Abstract: Endoplasmic reticulum-localized acyl-CoA:cholesterol acyltransferases (ACAT), including ACAT1 and ACAT2, convert cholesterol to cholesteryl esters that become incorporated into lipoproteins or stored ...Endoplasmic reticulum-localized acyl-CoA:cholesterol acyltransferases (ACAT), including ACAT1 and ACAT2, convert cholesterol to cholesteryl esters that become incorporated into lipoproteins or stored in cytosolic lipid droplets. Selective inhibition of ACAT2 has been shown to considerably attenuate hypercholesterolemia and atherosclerosis in mice. Here, we report cryogenic electron microscopy structures of human ACAT2 bound to its specific inhibitor pyripyropene A or the general ACAT inhibitor nevanimibe. Structural analysis reveals that ACAT2 has a topology in membranes similar to that of ACAT1. A catalytic core with an entry site occupied by a cholesterol molecule and another site for allosteric activation of ACAT2 is observed in these structures. Enzymatic assays show that mutations within sites of cholesterol entry or allosteric activation attenuate ACAT2 activity in vitro. Together, these results reveal mechanisms for ACAT2-mediated esterification of cholesterol, providing a blueprint to design new ACAT2 inhibitors for use in the prevention of cardiovascular disease. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_24208.map.gz emd_24208.map.gz | 26.7 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-24208-v30.xml emd-24208-v30.xml emd-24208.xml emd-24208.xml | 9.5 KB 9.5 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_24208.png emd_24208.png | 35.5 KB | ||
| Filedesc metadata |  emd-24208.cif.gz emd-24208.cif.gz | 5.1 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-24208 http://ftp.pdbj.org/pub/emdb/structures/EMD-24208 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-24208 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-24208 | HTTPS FTP |
-Validation report
| Summary document |  emd_24208_validation.pdf.gz emd_24208_validation.pdf.gz | 389 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_24208_full_validation.pdf.gz emd_24208_full_validation.pdf.gz | 388.6 KB | Display | |
| Data in XML |  emd_24208_validation.xml.gz emd_24208_validation.xml.gz | 7 KB | Display | |
| Data in CIF |  emd_24208_validation.cif.gz emd_24208_validation.cif.gz | 8 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-24208 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-24208 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-24208 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-24208 | HTTPS FTP |
-Related structure data
| Related structure data |  7n6qMC  7n6rC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_24208.map.gz / Format: CCP4 / Size: 137.1 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_24208.map.gz / Format: CCP4 / Size: 137.1 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Structure of PPPA bound human ACAT2 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.833 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
- Sample components
Sample components
-Entire : human ACAT2 with PPPA
| Entire | Name: human ACAT2 with PPPA |
|---|---|
| Components |
|
-Supramolecule #1: human ACAT2 with PPPA
| Supramolecule | Name: human ACAT2 with PPPA / type: organelle_or_cellular_component / ID: 1 / Parent: 0 / Macromolecule list: #1 |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
-Macromolecule #1: Sterol O-acyltransferase 2
| Macromolecule | Name: Sterol O-acyltransferase 2 / type: protein_or_peptide / ID: 1 / Number of copies: 4 / Enantiomer: LEVO / EC number: sterol O-acyltransferase |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 60.893 KDa |
| Recombinant expression | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: MDYKDDDDKE PGGARLRLQR TGGLGGERER QPCGDGNTET HRAPDLVQWT RHMEAVKAQL LEQAQGQLRE LLDRAMREAI QSYPSQDKP LPPPPPGSLS RTQEPSLGKQ KVFIIRKSLL DELMEVQHFR TIYHMFIAGL CVFIISTLAI DFIDEGRLLL E FDLLIFSF ...String: MDYKDDDDKE PGGARLRLQR TGGLGGERER QPCGDGNTET HRAPDLVQWT RHMEAVKAQL LEQAQGQLRE LLDRAMREAI QSYPSQDKP LPPPPPGSLS RTQEPSLGKQ KVFIIRKSLL DELMEVQHFR TIYHMFIAGL CVFIISTLAI DFIDEGRLLL E FDLLIFSF GQLPLALVTW VPMFLSTLLA PYQALRLWAR GTWTQATGLG CALLAAHAVV LCALPVHVAV EHQLPPASRC VL VFEQVRF LMKSYSFLRE AVPGILRARR GEGIQAPSFS SYLYFLFCPT LIYRETYPRT PYVRWNYVAK NFAQALGCVL YAC FILGRL CVPVFANMSR EPFSTRALVL SILHATLPGI FMLLLIFFAF LHCWLNAFAE MLRFGDRMFY RDWWNSTSFS NYYR TWNVV VHDWLYSYVY QDGLRLLGAR ARGVAMLGVF LVSAVAHEYI FCFVLGFFYP VMLILFLVIG GMLNFMMHDQ RTGPA WNVL MWTMLFLGQG IQVSLYCQEW YARRHCPLPQ ATFWGLVTPR SWSCHT UniProtKB: Sterol O-acyltransferase 2 |
-Macromolecule #2: (3S,4R,4aR,6S,6aS,12R,12aS,12bS)-4-[(acetyloxy)methyl]-12-hydroxy...
| Macromolecule | Name: (3S,4R,4aR,6S,6aS,12R,12aS,12bS)-4-[(acetyloxy)methyl]-12-hydroxy-4,6a,12b-trimethyl-11-oxo-9-(pyridin-3-yl)-1,3,4,4a,5,6,6a,12,12a,12b-decahydro-2H,11H-naphtho[2,1-b]pyrano[3,4-e]pyran-3,6-diyl diacetate type: ligand / ID: 2 / Number of copies: 4 / Formula: 7T8 |
|---|---|
| Molecular weight | Theoretical: 583.626 Da |
| Chemical component information | 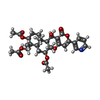 ChemComp-7T8: |
-Macromolecule #3: CHOLESTEROL
| Macromolecule | Name: CHOLESTEROL / type: ligand / ID: 3 / Number of copies: 8 / Formula: CLR |
|---|---|
| Molecular weight | Theoretical: 386.654 Da |
| Chemical component information |  ChemComp-CLR: |
-Macromolecule #4: OLEIC ACID
| Macromolecule | Name: OLEIC ACID / type: ligand / ID: 4 / Number of copies: 4 / Formula: OLA |
|---|---|
| Molecular weight | Theoretical: 282.461 Da |
| Chemical component information |  ChemComp-OLA: |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.5 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Average electron dose: 60.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: DARK FIELD |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
- Image processing
Image processing
| Startup model | Type of model: PDB ENTRY |
|---|---|
| Final reconstruction | Resolution.type: BY AUTHOR / Resolution: 3.87 Å / Resolution method: FSC 0.143 CUT-OFF / Number images used: 153208 |
| Initial angle assignment | Type: ANGULAR RECONSTITUTION |
| Final angle assignment | Type: ANGULAR RECONSTITUTION |
 Movie
Movie Controller
Controller














 X (Sec.)
X (Sec.) Y (Row.)
Y (Row.) Z (Col.)
Z (Col.)





















