+ Open data
Open data
- Basic information
Basic information
| Entry |  | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | Localized reconstruction of EEEV | ||||||||||||||||||
 Map data Map data | EEEV_alone | ||||||||||||||||||
 Sample Sample |
| ||||||||||||||||||
| Biological species |   Eastern equine encephalitis virus Eastern equine encephalitis virus | ||||||||||||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 5.1 Å | ||||||||||||||||||
 Authors Authors | Chen C-L / Kuhn RJ / Klose T | ||||||||||||||||||
| Funding support |  United States, 5 items United States, 5 items
| ||||||||||||||||||
 Citation Citation |  Journal: Proc Natl Acad Sci U S A / Year: 2022 Journal: Proc Natl Acad Sci U S A / Year: 2022Title: Cryo-EM structures of alphavirus conformational intermediates in low pH-triggered prefusion states. Authors: Chun-Liang Chen / Thomas Klose / Chengqun Sun / Arthur S Kim / Geeta Buda / Michael G Rossmann / Michael S Diamond / William B Klimstra / Richard J Kuhn /  Abstract: Alphaviruses can cause severe human arthritis and encephalitis. During virus infection, structural changes of viral glycoproteins in the acidified endosome trigger virus-host membrane fusion for ...Alphaviruses can cause severe human arthritis and encephalitis. During virus infection, structural changes of viral glycoproteins in the acidified endosome trigger virus-host membrane fusion for delivery of the capsid core and RNA genome into the cytosol to initiate virus translation and replication. However, mechanisms by which E1 and E2 glycoproteins rearrange in this process remain unknown. Here, we investigate prefusion cryoelectron microscopy (cryo-EM) structures of eastern equine encephalitis virus (EEEV) under acidic conditions. With models fitted into the low-pH cryo-EM maps, we suggest that E2 dissociates from E1, accompanied by a rotation (∼60°) of the E2-B domain (E2-B) to expose E1 fusion loops. Cryo-EM reconstructions of EEEV bound to a protective antibody at acidic and neutral pH suggest that stabilization of E2-B prevents dissociation of E2 from E1. These findings reveal conformational changes of the glycoprotein spikes in the acidified host endosome. Stabilization of E2-B may provide a strategy for antiviral agent development. | ||||||||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_24199.map.gz emd_24199.map.gz | 58.5 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-24199-v30.xml emd-24199-v30.xml emd-24199.xml emd-24199.xml | 13.4 KB 13.4 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_24199_fsc.xml emd_24199_fsc.xml | 9.1 KB | Display |  FSC data file FSC data file |
| Images |  emd_24199.png emd_24199.png | 111 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-24199 http://ftp.pdbj.org/pub/emdb/structures/EMD-24199 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-24199 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-24199 | HTTPS FTP |
-Validation report
| Summary document |  emd_24199_validation.pdf.gz emd_24199_validation.pdf.gz | 482.2 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_24199_full_validation.pdf.gz emd_24199_full_validation.pdf.gz | 481.8 KB | Display | |
| Data in XML |  emd_24199_validation.xml.gz emd_24199_validation.xml.gz | 11.3 KB | Display | |
| Data in CIF |  emd_24199_validation.cif.gz emd_24199_validation.cif.gz | 14.7 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-24199 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-24199 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-24199 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-24199 | HTTPS FTP |
-Related structure data
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_24199.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_24199.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | EEEV_alone | ||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.618 Å | ||||||||||||||||||||||||||||||||||||
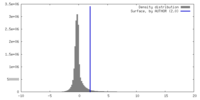
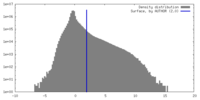
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
- Sample components
Sample components
-Entire : Eastern equine encephalitis virus
| Entire | Name:   Eastern equine encephalitis virus Eastern equine encephalitis virus |
|---|---|
| Components |
|
-Supramolecule #1: Eastern equine encephalitis virus
| Supramolecule | Name: Eastern equine encephalitis virus / type: virus / ID: 1 / Parent: 0 / NCBI-ID: 11021 / Sci species name: Eastern equine encephalitis virus / Virus type: VIRION / Virus isolate: STRAIN / Virus enveloped: Yes / Virus empty: No |
|---|---|
| Host (natural) | Organism:  |
| Host system | Organism:  Cricetinae gen. sp. (mammal) / Recombinant cell: Baby hamster kidney cells Cricetinae gen. sp. (mammal) / Recombinant cell: Baby hamster kidney cells |
| Virus shell | Shell ID: 1 / Diameter: 660.0 Å |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 0.2 mg/mL | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Buffer | pH: 8 Component:
Details: pH 8 | ||||||||||||
| Grid | Model: PELCO Ultrathin Carbon with Lacey Carbon / Support film - Material: CARBON / Support film - topology: CONTINUOUS / Pretreatment - Type: GLOW DISCHARGE / Pretreatment - Atmosphere: AIR / Details: 25 mA | ||||||||||||
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 80 % / Chamber temperature: 298 K / Instrument: GATAN CRYOPLUNGE 3 / Details: blot for 3.3 seconds before plunging. | ||||||||||||
| Details | Estimate E2 protein concentration by SDS-PAGE with BSA ranging from 0.1 to 1 microgram. |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Temperature | Min: 100.0 K |
| Image recording | Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Detector mode: SUPER-RESOLUTION / Number grids imaged: 1 / Average electron dose: 32.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | C2 aperture diameter: 100.0 µm / Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm / Nominal magnification: 38000 |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
+ Image processing
Image processing
-Atomic model buiding 1
| Initial model | PDB ID: |
|---|---|
| Refinement | Protocol: RIGID BODY FIT |
 Movie
Movie Controller
Controller











 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)