[English] 日本語
 Yorodumi
Yorodumi- EMDB-24134: Elongating 70S ribosome complex in a spectinomycin-stalled interm... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-24134 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Elongating 70S ribosome complex in a spectinomycin-stalled intermediate state of translocation bound to EF-G in an active, GTP conformation (INT1) | |||||||||
 Map data Map data | Post-processed map | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | Elongation complex / tRNA / mRNA / translocation / EF-G & GTP / RIBOSOME | |||||||||
| Function / homology |  Function and homology information Function and homology informationribosome disassembly / guanosine tetraphosphate binding / negative regulation of cytoplasmic translational initiation / stringent response / transcription antitermination factor activity, RNA binding / ornithine decarboxylase inhibitor activity / transcriptional attenuation / endoribonuclease inhibitor activity / translation elongation factor activity / positive regulation of ribosome biogenesis ...ribosome disassembly / guanosine tetraphosphate binding / negative regulation of cytoplasmic translational initiation / stringent response / transcription antitermination factor activity, RNA binding / ornithine decarboxylase inhibitor activity / transcriptional attenuation / endoribonuclease inhibitor activity / translation elongation factor activity / positive regulation of ribosome biogenesis / RNA-binding transcription regulator activity / translational termination / negative regulation of cytoplasmic translation / four-way junction DNA binding / DnaA-L2 complex / translation repressor activity / negative regulation of translational initiation / regulation of mRNA stability / negative regulation of DNA-templated DNA replication initiation / mRNA regulatory element binding translation repressor activity / assembly of large subunit precursor of preribosome / cytosolic ribosome assembly / response to reactive oxygen species / regulation of DNA-templated transcription elongation / ribosome assembly / transcription elongation factor complex / transcription antitermination / DNA endonuclease activity / regulation of cell growth / translational initiation / DNA-templated transcription termination / response to radiation / maintenance of translational fidelity / mRNA 5'-UTR binding / regulation of translation / large ribosomal subunit / ribosome biogenesis / transferase activity / ribosome binding / ribosomal small subunit biogenesis / ribosomal small subunit assembly / ribosomal large subunit assembly / 5S rRNA binding / small ribosomal subunit / small ribosomal subunit rRNA binding / cytosolic small ribosomal subunit / large ribosomal subunit rRNA binding / cytosolic large ribosomal subunit / cytoplasmic translation / tRNA binding / negative regulation of translation / rRNA binding / structural constituent of ribosome / ribosome / translation / hydrolase activity / response to antibiotic / negative regulation of DNA-templated transcription / GTPase activity / mRNA binding / GTP binding / DNA binding / RNA binding / zinc ion binding / membrane / cytoplasm / cytosol Similarity search - Function | |||||||||
| Biological species |   | |||||||||
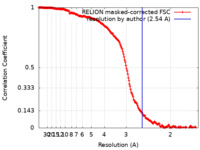
| Method | single particle reconstruction / cryo EM / Resolution: 2.54 Å | |||||||||
 Authors Authors | Rundlet EJ / Holm M | |||||||||
 Citation Citation |  Journal: Nature / Year: 2021 Journal: Nature / Year: 2021Title: Structural basis of early translocation events on the ribosome. Authors: Emily J Rundlet / Mikael Holm / Magdalena Schacherl / S Kundhavai Natchiar / Roger B Altman / Christian M T Spahn / Alexander G Myasnikov / Scott C Blanchard /   Abstract: Peptide-chain elongation during protein synthesis entails sequential aminoacyl-tRNA selection and translocation reactions that proceed rapidly (2-20 per second) and with a low error rate (around ...Peptide-chain elongation during protein synthesis entails sequential aminoacyl-tRNA selection and translocation reactions that proceed rapidly (2-20 per second) and with a low error rate (around 10 to 10 at each step) over thousands of cycles. The cadence and fidelity of ribosome transit through mRNA templates in discrete codon increments is a paradigm for movement in biological systems that must hold for diverse mRNA and tRNA substrates across domains of life. Here we use single-molecule fluorescence methods to guide the capture of structures of early translocation events on the bacterial ribosome. Our findings reveal that the bacterial GTPase elongation factor G specifically engages spontaneously achieved ribosome conformations while in an active, GTP-bound conformation to unlock and initiate peptidyl-tRNA translocation. These findings suggest that processes intrinsic to the pre-translocation ribosome complex can regulate the rate of protein synthesis, and that energy expenditure is used later in the translocation mechanism than previously proposed. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_24134.map.gz emd_24134.map.gz | 16.9 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-24134-v30.xml emd-24134-v30.xml emd-24134.xml emd-24134.xml | 89 KB 89 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_24134_fsc.xml emd_24134_fsc.xml | 18.1 KB | Display |  FSC data file FSC data file |
| Images |  emd_24134.png emd_24134.png | 61.3 KB | ||
| Filedesc metadata |  emd-24134.cif.gz emd-24134.cif.gz | 16.7 KB | ||
| Others |  emd_24134_additional_1.map.gz emd_24134_additional_1.map.gz emd_24134_half_map_1.map.gz emd_24134_half_map_1.map.gz emd_24134_half_map_2.map.gz emd_24134_half_map_2.map.gz | 218.6 MB 411.4 MB 410.6 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-24134 http://ftp.pdbj.org/pub/emdb/structures/EMD-24134 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-24134 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-24134 | HTTPS FTP |
-Related structure data
| Related structure data |  7n2vMC  7n1pC  7n2cC  7n2uC  7n30C  7n31C M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_24134.map.gz / Format: CCP4 / Size: 729 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_24134.map.gz / Format: CCP4 / Size: 729 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
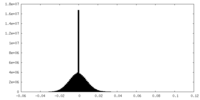
| Annotation | Post-processed map | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.06 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
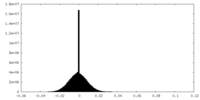
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
-Additional map: Masked refinement map
| File | emd_24134_additional_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Masked refinement map | ||||||||||||
| Projections & Slices |
| ||||||||||||
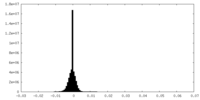
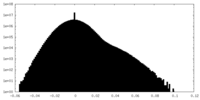
| Density Histograms |
-Half map: Half map
| File | emd_24134_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Half map | ||||||||||||
| Projections & Slices |
| ||||||||||||
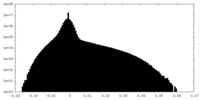
| Density Histograms |
-Half map: Half map
| File | emd_24134_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Half map | ||||||||||||
| Projections & Slices |
| ||||||||||||
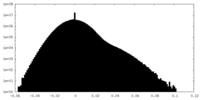
| Density Histograms |
- Sample components
Sample components
+Entire : Elongating 70S ribosome complex in a spectinomycin-stalled interm...
+Supramolecule #1: Elongating 70S ribosome complex in a spectinomycin-stalled interm...
+Macromolecule #1: 16S rRNA
+Macromolecule #22: mRNA
+Macromolecule #23: 23S rRNA
+Macromolecule #24: 5S rRNA
+Macromolecule #58: tRNA
+Macromolecule #59: tRNA
+Macromolecule #2: 30S ribosomal protein S2
+Macromolecule #3: 30S ribosomal protein S3
+Macromolecule #4: 30S ribosomal protein S4
+Macromolecule #5: 30S ribosomal protein S5
+Macromolecule #6: 30S ribosomal protein S6
+Macromolecule #7: 30S ribosomal protein S7
+Macromolecule #8: 30S ribosomal protein S8
+Macromolecule #9: 30S ribosomal protein S9
+Macromolecule #10: 30S ribosomal protein S10
+Macromolecule #11: 30S ribosomal protein S11
+Macromolecule #12: 30S ribosomal protein S12
+Macromolecule #13: 30S ribosomal protein S13
+Macromolecule #14: 30S ribosomal protein S14
+Macromolecule #15: 30S ribosomal protein S15
+Macromolecule #16: 30S ribosomal protein S16
+Macromolecule #17: 30S ribosomal protein S17
+Macromolecule #18: 30S ribosomal protein S18
+Macromolecule #19: 30S ribosomal protein S19
+Macromolecule #20: 30S ribosomal protein S20
+Macromolecule #21: 30S ribosomal protein S21
+Macromolecule #25: 50S ribosomal protein L2
+Macromolecule #26: 50S ribosomal protein L3
+Macromolecule #27: 50S ribosomal protein L4
+Macromolecule #28: 50S ribosomal protein L5
+Macromolecule #29: 50S ribosomal protein L6
+Macromolecule #30: 50S ribosomal protein L9
+Macromolecule #31: 50S ribosomal protein L10
+Macromolecule #32: 50S ribosomal protein L11
+Macromolecule #33: 50S ribosomal protein L13
+Macromolecule #34: 50S ribosomal protein L14
+Macromolecule #35: 50S ribosomal protein L15
+Macromolecule #36: 50S ribosomal protein L16
+Macromolecule #37: 50S ribosomal protein L17
+Macromolecule #38: 50S ribosomal protein L18
+Macromolecule #39: 50S ribosomal protein L19
+Macromolecule #40: 50S ribosomal protein L20
+Macromolecule #41: 50S ribosomal protein L21
+Macromolecule #42: 50S ribosomal protein L22
+Macromolecule #43: 50S ribosomal protein L23
+Macromolecule #44: 50S ribosomal protein L24
+Macromolecule #45: 50S ribosomal protein L25
+Macromolecule #46: 50S ribosomal protein L27
+Macromolecule #47: 50S ribosomal protein L28
+Macromolecule #48: 50S ribosomal protein L29
+Macromolecule #49: 50S ribosomal protein L30
+Macromolecule #50: 50S ribosomal protein L31
+Macromolecule #51: 50S ribosomal protein L32
+Macromolecule #52: 50S ribosomal protein L33
+Macromolecule #53: 50S ribosomal protein L34
+Macromolecule #54: 50S ribosomal protein L35
+Macromolecule #55: 50S ribosomal protein L36
+Macromolecule #56: Elongation factor G
+Macromolecule #57: Nascent peptide
+Macromolecule #60: SPECTINOMYCIN
+Macromolecule #61: 1,4-DIAMINOBUTANE
+Macromolecule #62: MAGNESIUM ION
+Macromolecule #63: ZINC ION
+Macromolecule #64: ADENOSINE-5'-TRIPHOSPHATE
+Macromolecule #65: SPERMIDINE
+Macromolecule #66: GUANOSINE-5'-TRIPHOSPHATE
+Macromolecule #67: water
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 5.5 mg/mL |
|---|---|
| Buffer | pH: 7.5 |
| Grid | Model: Quantifoil R1.2/1.3 / Material: COPPER / Mesh: 300 / Pretreatment - Type: GLOW DISCHARGE / Pretreatment - Time: 20 sec. / Pretreatment - Atmosphere: OTHER |
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 95 % / Chamber temperature: 283 K / Instrument: FEI VITROBOT MARK IV |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Average electron dose: 87.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
+ Image processing
Image processing
-Atomic model buiding 1
| Refinement | Space: RECIPROCAL / Protocol: OTHER |
|---|---|
| Output model |  PDB-7n2v: |
 Movie
Movie Controller
Controller


























 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)