[English] 日本語
 Yorodumi
Yorodumi- EMDB-23898: SARS-CoV-2 Spike in complex with neutralizing Fab SARS2-38 (three... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-23898 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | SARS-CoV-2 Spike in complex with neutralizing Fab SARS2-38 (three down conformation) | |||||||||
 Map data Map data | Map of SARS2-38 antibody Fab bound to SARS-CoV-2 spike | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | Glycoprotein / Antibody / VIRAL PROTEIN-IMMUNE SYSTEM complex / Structural Genomics / Center for Structural Genomics of Infectious Diseases / CSGID | |||||||||
| Function / homology |  Function and homology information Function and homology informationsymbiont-mediated disruption of host tissue / Maturation of spike protein / Translation of Structural Proteins / Virion Assembly and Release / host cell surface / viral translation / host extracellular space / symbiont-mediated-mediated suppression of host tetherin activity / Induction of Cell-Cell Fusion / structural constituent of virion ...symbiont-mediated disruption of host tissue / Maturation of spike protein / Translation of Structural Proteins / Virion Assembly and Release / host cell surface / viral translation / host extracellular space / symbiont-mediated-mediated suppression of host tetherin activity / Induction of Cell-Cell Fusion / structural constituent of virion / membrane fusion / entry receptor-mediated virion attachment to host cell / Attachment and Entry / host cell endoplasmic reticulum-Golgi intermediate compartment membrane / positive regulation of viral entry into host cell / receptor-mediated virion attachment to host cell / host cell surface receptor binding / symbiont-mediated suppression of host innate immune response / receptor ligand activity / endocytosis involved in viral entry into host cell / fusion of virus membrane with host plasma membrane / fusion of virus membrane with host endosome membrane / viral envelope / symbiont entry into host cell / virion attachment to host cell / SARS-CoV-2 activates/modulates innate and adaptive immune responses / host cell plasma membrane / virion membrane / identical protein binding / membrane / plasma membrane Similarity search - Function | |||||||||
| Biological species |   | |||||||||
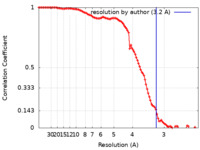
| Method | single particle reconstruction / cryo EM / Resolution: 3.2 Å | |||||||||
 Authors Authors | Adams LJ / Fremont DH | |||||||||
| Funding support |  United States, 2 items United States, 2 items
| |||||||||
 Citation Citation | Journal: bioRxiv / Year: 2021 Title: A potently neutralizing anti-SARS-CoV-2 antibody inhibits variants of concern by binding a highly conserved epitope. Authors: Laura VanBlargan / Lucas Adams / Zhuoming Liu / Rita E Chen / Pavlo Gilchuk / Saravanan Raju / Brittany Smith / Haiyan Zhao / James Brett Case / Emma S Winkler / Bradley Whitener / Lindsay ...Authors: Laura VanBlargan / Lucas Adams / Zhuoming Liu / Rita E Chen / Pavlo Gilchuk / Saravanan Raju / Brittany Smith / Haiyan Zhao / James Brett Case / Emma S Winkler / Bradley Whitener / Lindsay Droit / Ismael Aziati / Pei-Yong Shi / Adrian Creanga / Amarendra Pegu / Scott Handley / David Wang / Adrianus Boon / James E Crowe / Sean P J Whelan / Daved Fremont / Michael Diamond Abstract: With the emergence of SARS-CoV-2 variants with increased transmissibility and potential resistance, antibodies and vaccines with broadly inhibitory activity are needed. Here we developed a panel of ...With the emergence of SARS-CoV-2 variants with increased transmissibility and potential resistance, antibodies and vaccines with broadly inhibitory activity are needed. Here we developed a panel of neutralizing anti-SARS-CoV-2 mAbs that bind the receptor binding domain of the spike protein at distinct epitopes and block virus attachment to cells and its receptor, human angiotensin converting enzyme-2 (hACE2). While several potently neutralizing mAbs protected K18-hACE2 transgenic mice against infection caused by historical SARS-CoV-2 strains, others induced escape variants in vivo and lost activity against emerging strains. We identified one mAb, SARS2-38, that potently neutralizes all SARS-CoV-2 variants of concern tested and protects mice against challenge by multiple SARS-CoV-2 strains. Structural analysis showed that SARS2-38 engages a conserved epitope proximal to the receptor binding motif. Thus, treatment with or induction of inhibitory antibodies that bind conserved spike epitopes may limit the loss of potency of therapies or vaccines against emerging SARS-CoV-2 variants. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_23898.map.gz emd_23898.map.gz | 91.8 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-23898-v30.xml emd-23898-v30.xml emd-23898.xml emd-23898.xml | 23.9 KB 23.9 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_23898_fsc.xml emd_23898_fsc.xml | 10.4 KB | Display |  FSC data file FSC data file |
| Images |  emd_23898.png emd_23898.png | 83.8 KB | ||
| Filedesc metadata |  emd-23898.cif.gz emd-23898.cif.gz | 8.2 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-23898 http://ftp.pdbj.org/pub/emdb/structures/EMD-23898 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-23898 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-23898 | HTTPS FTP |
-Validation report
| Summary document |  emd_23898_validation.pdf.gz emd_23898_validation.pdf.gz | 548.7 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_23898_full_validation.pdf.gz emd_23898_full_validation.pdf.gz | 548.3 KB | Display | |
| Data in XML |  emd_23898_validation.xml.gz emd_23898_validation.xml.gz | 11.7 KB | Display | |
| Data in CIF |  emd_23898_validation.cif.gz emd_23898_validation.cif.gz | 15.7 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-23898 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-23898 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-23898 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-23898 | HTTPS FTP |
-Related structure data
| Related structure data |  7mklMC  7mkmC C: citing same article ( M: atomic model generated by this map |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_23898.map.gz / Format: CCP4 / Size: 103 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_23898.map.gz / Format: CCP4 / Size: 103 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Map of SARS2-38 antibody Fab bound to SARS-CoV-2 spike | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.16 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
- Sample components
Sample components
-Entire : SARS-CoV-2 spike bound by SARS2-38 antibody Fab
| Entire | Name: SARS-CoV-2 spike bound by SARS2-38 antibody Fab |
|---|---|
| Components |
|
-Supramolecule #1: SARS-CoV-2 spike bound by SARS2-38 antibody Fab
| Supramolecule | Name: SARS-CoV-2 spike bound by SARS2-38 antibody Fab / type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1-#3 |
|---|---|
| Source (natural) | Organism:  |
-Macromolecule #1: Spike glycoprotein
| Macromolecule | Name: Spike glycoprotein / type: protein_or_peptide / ID: 1 / Number of copies: 3 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 124.152539 KDa |
| Recombinant expression | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: AYTNSFTRGV YYPDKVFRSS VLHSTQDLFL PFFSNVTWFH AIHVSGTNGT KRFDNPVLPF NDGVYFASTE KSNIIRGWIF GTTLDSKTQ SLLIVNNATN VVIKVCEFQF CNDPFLGVYY HKNNKSWMES EFRVYSSANN CTFEYVSQPF LMDLEGKQGN F KNLREFVF ...String: AYTNSFTRGV YYPDKVFRSS VLHSTQDLFL PFFSNVTWFH AIHVSGTNGT KRFDNPVLPF NDGVYFASTE KSNIIRGWIF GTTLDSKTQ SLLIVNNATN VVIKVCEFQF CNDPFLGVYY HKNNKSWMES EFRVYSSANN CTFEYVSQPF LMDLEGKQGN F KNLREFVF KNIDGYFKIY SKHTPINLVR DLPQGFSALE PLVDLPIGIN ITRFQTLLAL HRSYLTPGDS SSGWTAGAAA YY VGYLQPR TFLLKYNENG TITDAVDCAL DPLSETKCTL KSFTVEKGIY QTSNFRVQPT ESIVRFPNIT NLCPFGEVFN ATR FASVYA WNRKRISNCV ADYSVLYNSA SFSTFKCYGV SPTKLNDLCF TNVYADSFVI RGDEVRQIAP GQTGKIADYN YKLP DDFTG CVIAWNSNNL DSKVGGNYNY LYRLFRKSNL KPFERDISTE IYQAGSTPCN GVEGFNCYFP LQSYGFQPTN GVGYQ PYRV VVLSFELLHA PATVCGPKKS TNLVKNKCVN FNFNGLTGTG VLTESNKKFL PFQQFGRDIA DTTDAVRDPQ TLEILD ITP CSFGGVSVIT PGTNTSNQVA VLYQDVNCTE VPVAIHADQL TPTWRVYSTG SNVFQTRAGC LIGAEHVNNS YECDIPI GA GICASYQTQT NSPRRARSVA SQSIIAYTMS LGAENSVAYS NNSIAIPTNF TISVTTEILP VSMTKTSVDC TMYICGDS T ECSNLLLQYG SFCTQLNRAL TGIAVEQDKN TQEVFAQVKQ IYKTPPIKDF GGFNFSQILP DPSKPSKRSP IEDLLFNKV TLADAGFIKQ YGDCLGDIAA RDLICAQKFN GLTVLPPLLT DEMIAQYTSA LLAGTITSGW TFGAGPALQI PFPMQMAYRF NGIGVTQNV LYENQKLIAN QFNSAIGKIQ DSLSSTPSAL GKLQDVVNQN AQALNTLVKQ LSSNFGAISS VLNDILSRLD P PEAEVQID RLITGRLQSL QTYVTQQLIR AAEIRASANL AATKMSECVL GQSKRVDFCG KGYHLMSFPQ SAPHGVVFLH VT YVPAQEK NFTTAPAICH DGKAHFPREG VFVSNGTHWF VTQRNFYEPQ IITTDNTFVS GNCDVVIGIV NNTVYDPLQP ELD S UniProtKB: Spike glycoprotein |
-Macromolecule #2: SARS2-38 Fv heavy chain
| Macromolecule | Name: SARS2-38 Fv heavy chain / type: protein_or_peptide / ID: 2 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 12.490882 KDa |
| Sequence | String: QVQLKESGPG LVAPSQSLSI TCTVSGFSLT RYGVHWVRQP PGKGLEWLGV IWADGSTYYN SALMSRLSIS KDNSKSQVFL NMNSLQTDD TAKYYCARDG RGYDDYWGQG TTLT |
-Macromolecule #3: SARS2-38 Fv light chain
| Macromolecule | Name: SARS2-38 Fv light chain / type: protein_or_peptide / ID: 3 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 11.419787 KDa |
| Sequence | String: QIVLTQSPAI MSASPGEKVT MTCSASSTVS FIYWYQQKPG SSPRLLIYDT SNPASGVPVR FSGSGCGTSY YLTISRMEAE DAATYYCQQ WNTYPLTFGA GTKLEL |
-Macromolecule #5: 2-acetamido-2-deoxy-beta-D-glucopyranose
| Macromolecule | Name: 2-acetamido-2-deoxy-beta-D-glucopyranose / type: ligand / ID: 5 / Number of copies: 33 / Formula: NAG |
|---|---|
| Molecular weight | Theoretical: 221.208 Da |
| Chemical component information |  ChemComp-NAG: |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.4 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: FEI FALCON IV (4k x 4k) / Average electron dose: 50.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller














 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)