+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Structure of EC+EC (leading EC-focused) | |||||||||
 Map data Map data | Leading EC_map_with_sharpening by postprocesssed in Relion | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | pol II / TRANSCRIPTION | |||||||||
| Function / homology |  Function and homology information Function and homology information: / : / : / : / : / positive regulation of nuclear-transcribed mRNA poly(A) tail shortening / maintenance of transcriptional fidelity during transcription elongation by RNA polymerase II / positive regulation of translational initiation / RNA polymerase I complex / RNA polymerase III complex ...: / : / : / : / : / positive regulation of nuclear-transcribed mRNA poly(A) tail shortening / maintenance of transcriptional fidelity during transcription elongation by RNA polymerase II / positive regulation of translational initiation / RNA polymerase I complex / RNA polymerase III complex / RNA polymerase II, core complex / tRNA transcription by RNA polymerase III / transcription-coupled nucleotide-excision repair / translation initiation factor binding / transcription initiation at RNA polymerase II promoter / P-body / ribonucleoside binding / DNA-directed RNA polymerase / DNA-directed RNA polymerase activity / single-stranded DNA binding / nucleic acid binding / protein dimerization activity / single-stranded RNA binding / nucleotide binding / DNA-templated transcription / nucleolus / DNA binding / zinc ion binding / metal ion binding Similarity search - Function | |||||||||
| Biological species |  | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 3.54 Å | |||||||||
 Authors Authors | Yang C / Murakami K | |||||||||
| Funding support | 1 items
| |||||||||
 Citation Citation |  Journal: Mol Cell / Year: 2022 Journal: Mol Cell / Year: 2022Title: Structural visualization of de novo transcription initiation by Saccharomyces cerevisiae RNA polymerase II. Authors: Chun Yang / Rina Fujiwara / Hee Jong Kim / Pratik Basnet / Yunye Zhu / Jose J Gorbea Colón / Stefan Steimle / Benjamin A Garcia / Craig D Kaplan / Kenji Murakami /  Abstract: Previous structural studies of the initiation-elongation transition of RNA polymerase II (pol II) transcription have relied on the use of synthetic oligonucleotides, often artificially discontinuous ...Previous structural studies of the initiation-elongation transition of RNA polymerase II (pol II) transcription have relied on the use of synthetic oligonucleotides, often artificially discontinuous to capture pol II in the initiating state. Here, we report multiple structures of initiation complexes converted de novo from a 33-subunit yeast pre-initiation complex (PIC) through catalytic activities and subsequently stalled at different template positions. We determine that PICs in the initially transcribing complex (ITC) can synthesize a transcript of ∼26 nucleotides before transitioning to an elongation complex (EC) as determined by the loss of general transcription factors (GTFs). Unexpectedly, transition to an EC was greatly accelerated when an ITC encountered a downstream EC stalled at promoter proximal regions and resulted in a collided head-to-end dimeric EC complex. Our structural analysis reveals a dynamic state of TFIIH, the largest of GTFs, in PIC/ITC with distinct functional consequences at multiple steps on the pathway to elongation. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_23888.map.gz emd_23888.map.gz | 9.9 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-23888-v30.xml emd-23888-v30.xml emd-23888.xml emd-23888.xml | 35.8 KB 35.8 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_23888_fsc.xml emd_23888_fsc.xml | 7.9 KB | Display |  FSC data file FSC data file |
| Images |  emd_23888.png emd_23888.png | 67.9 KB | ||
| Masks |  emd_23888_msk_1.map emd_23888_msk_1.map | 40.6 MB |  Mask map Mask map | |
| Filedesc metadata |  emd-23888.cif.gz emd-23888.cif.gz | 9.7 KB | ||
| Others |  emd_23888_additional_1.map.gz emd_23888_additional_1.map.gz emd_23888_half_map_1.map.gz emd_23888_half_map_1.map.gz emd_23888_half_map_2.map.gz emd_23888_half_map_2.map.gz | 31.2 MB 31.3 MB 31.3 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-23888 http://ftp.pdbj.org/pub/emdb/structures/EMD-23888 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-23888 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-23888 | HTTPS FTP |
-Related structure data
| Related structure data |  7mkaMC  7meiC  7mk9C  7ml0C  7ml1C  7ml2C  7ml3C  7ml4C M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_23888.map.gz / Format: CCP4 / Size: 40.6 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_23888.map.gz / Format: CCP4 / Size: 40.6 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Leading EC_map_with_sharpening by postprocesssed in Relion | ||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.08 Å | ||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Mask #1
| File |  emd_23888_msk_1.map emd_23888_msk_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
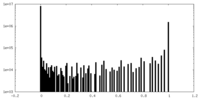
| Density Histograms |
-Additional map: Leading EC map without sharpening
| File | emd_23888_additional_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Leading EC_map_without_sharpening | ||||||||||||
| Projections & Slices |
| ||||||||||||
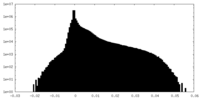
| Density Histograms |
-Half map: EC+EC (leading EC-focused) half map 1
| File | emd_23888_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | EC+EC (leading EC-focused) half map 1 | ||||||||||||
| Projections & Slices |
| ||||||||||||
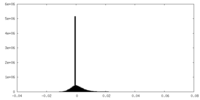
| Density Histograms |
-Half map: EC+EC (leading EC-focused) half map 2
| File | emd_23888_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | EC+EC (leading EC-focused) half map 2 | ||||||||||||
| Projections & Slices |
| ||||||||||||
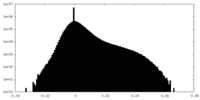
| Density Histograms |
- Sample components
Sample components
+Entire : The leading EC of complex EC+EC
+Supramolecule #1: The leading EC of complex EC+EC
+Macromolecule #1: DNA (40-MER)
+Macromolecule #2: DNA (40-MER)
+Macromolecule #3: DNA-directed RNA polymerase subunit
+Macromolecule #4: DNA-directed RNA polymerase subunit beta
+Macromolecule #5: DNA-directed RNA polymerase II subunit RPB3
+Macromolecule #6: DNA-directed RNA polymerase II subunit RPB4
+Macromolecule #7: DNA-directed RNA polymerases I, II, and III subunit RPABC1
+Macromolecule #8: DNA-directed RNA polymerases I,II,and III subunit RPABC2
+Macromolecule #9: DNA-directed RNA polymerase II subunit RPB7
+Macromolecule #10: DNA-directed RNA polymerases I, II, and III subunit RPABC3
+Macromolecule #11: DNA-directed RNA polymerase II subunit RPB9
+Macromolecule #12: DNA-directed RNA polymerases II subunit RPABC5
+Macromolecule #13: DNA-directed RNA polymerase II subunit RPB11
+Macromolecule #14: DNA-directed RNA polymerases II subunit RPABC4
+Macromolecule #15: RNA (5'-R(P*AP*AP*CP*UP*AP*GP*UP*UP*AP*AP*GP*AP*GP*GP*UP*U)-3')
+Macromolecule #16: ZINC ION
+Macromolecule #17: MAGNESIUM ION
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 1.0 mg/mL |
|---|---|
| Buffer | pH: 7.6 / Details: 20 mM HEPES PH 7.6 50 mM KoAc 5 mM DTT 2 mM MgoAc |
| Grid | Model: Quantifoil R1.2/1.3 / Material: COPPER |
| Vitrification | Cryogen name: ETHANE / Instrument: LEICA EM CPC |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Average electron dose: 1.25 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | C2 aperture diameter: 100.0 µm / Illumination mode: SPOT SCAN / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm / Nominal defocus max: 1.75 µm / Nominal defocus min: 0.75 µm / Nominal magnification: 81000 |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
+ Image processing
Image processing
-Atomic model buiding 1
| Initial model |
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Refinement | Space: REAL / Protocol: RIGID BODY FIT | ||||||||
| Output model |  PDB-7mka: |
 Movie
Movie Controller
Controller

















 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)