[English] 日本語
 Yorodumi
Yorodumi- EMDB-23694: Structure of the SARS-CoV-2 S 6P trimer in complex with the human... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-23694 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Structure of the SARS-CoV-2 S 6P trimer in complex with the human neutralizing antibody Fab fragment, BG1-22 | |||||||||
 Map data Map data | Sharpened map | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | SARS-CoV-2 / Coronavirus / COVID-19 / antibody / neutralizing antibody / receptor binding domain / spike glycoprotein / ANTIVIRAL PROTEIN / VIRAL PROTEIN-ANTIVIRAL PROTEIN complex | |||||||||
| Function / homology |  Function and homology information Function and homology informationsymbiont-mediated disruption of host tissue / Maturation of spike protein / Translation of Structural Proteins / Virion Assembly and Release / host cell surface / viral translation / host extracellular space / symbiont-mediated-mediated suppression of host tetherin activity / Induction of Cell-Cell Fusion / structural constituent of virion ...symbiont-mediated disruption of host tissue / Maturation of spike protein / Translation of Structural Proteins / Virion Assembly and Release / host cell surface / viral translation / host extracellular space / symbiont-mediated-mediated suppression of host tetherin activity / Induction of Cell-Cell Fusion / structural constituent of virion / entry receptor-mediated virion attachment to host cell / membrane fusion / Attachment and Entry / host cell endoplasmic reticulum-Golgi intermediate compartment membrane / positive regulation of viral entry into host cell / receptor-mediated virion attachment to host cell / host cell surface receptor binding / symbiont-mediated suppression of host innate immune response / receptor ligand activity / endocytosis involved in viral entry into host cell / fusion of virus membrane with host plasma membrane / fusion of virus membrane with host endosome membrane / viral envelope / symbiont entry into host cell / virion attachment to host cell / SARS-CoV-2 activates/modulates innate and adaptive immune responses / host cell plasma membrane / virion membrane / identical protein binding / membrane / plasma membrane Similarity search - Function | |||||||||
| Biological species |   Homo sapiens (human) Homo sapiens (human) | |||||||||
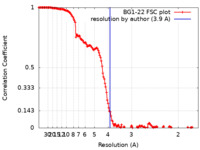
| Method | single particle reconstruction / cryo EM / Resolution: 3.9 Å | |||||||||
 Authors Authors | Barnes CO / Bjorkman PJ | |||||||||
| Funding support |  United States, 2 items United States, 2 items
| |||||||||
 Citation Citation |  Journal: Cell / Year: 2021 Journal: Cell / Year: 2021Title: B cell genomics behind cross-neutralization of SARS-CoV-2 variants and SARS-CoV. Authors: Johannes F Scheid / Christopher O Barnes / Basak Eraslan / Andrew Hudak / Jennifer R Keeffe / Lisa A Cosimi / Eric M Brown / Frauke Muecksch / Yiska Weisblum / Shuting Zhang / Toni Delorey / ...Authors: Johannes F Scheid / Christopher O Barnes / Basak Eraslan / Andrew Hudak / Jennifer R Keeffe / Lisa A Cosimi / Eric M Brown / Frauke Muecksch / Yiska Weisblum / Shuting Zhang / Toni Delorey / Ann E Woolley / Fadi Ghantous / Sung-Moo Park / Devan Phillips / Betsabeh Tusi / Kathryn E Huey-Tubman / Alexander A Cohen / Priyanthi N P Gnanapragasam / Kara Rzasa / Theodora Hatziioanno / Michael A Durney / Xiebin Gu / Takuya Tada / Nathaniel R Landau / Anthony P West / Orit Rozenblatt-Rosen / Michael S Seaman / Lindsey R Baden / Daniel B Graham / Jacques Deguine / Paul D Bieniasz / Aviv Regev / Deborah Hung / Pamela J Bjorkman / Ramnik J Xavier /  Abstract: Monoclonal antibodies (mAbs) are a focus in vaccine and therapeutic design to counteract severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and its variants. Here, we combined B cell ...Monoclonal antibodies (mAbs) are a focus in vaccine and therapeutic design to counteract severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and its variants. Here, we combined B cell sorting with single-cell VDJ and RNA sequencing (RNA-seq) and mAb structures to characterize B cell responses against SARS-CoV-2. We show that the SARS-CoV-2-specific B cell repertoire consists of transcriptionally distinct B cell populations with cells producing potently neutralizing antibodies (nAbs) localized in two clusters that resemble memory and activated B cells. Cryo-electron microscopy structures of selected nAbs from these two clusters complexed with SARS-CoV-2 spike trimers show recognition of various receptor-binding domain (RBD) epitopes. One of these mAbs, BG10-19, locks the spike trimer in a closed conformation to potently neutralize SARS-CoV-2, the recently arising mutants B.1.1.7 and B.1.351, and SARS-CoV and cross-reacts with heterologous RBDs. Together, our results characterize transcriptional differences among SARS-CoV-2-specific B cells and uncover cross-neutralizing Ab targets that will inform immunogen and therapeutic design against coronaviruses. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_23694.map.gz emd_23694.map.gz | 290.4 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-23694-v30.xml emd-23694-v30.xml emd-23694.xml emd-23694.xml | 25 KB 25 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_23694_fsc.xml emd_23694_fsc.xml | 15.7 KB | Display |  FSC data file FSC data file |
| Images |  emd_23694.png emd_23694.png | 28.1 KB | ||
| Filedesc metadata |  emd-23694.cif.gz emd-23694.cif.gz | 8 KB | ||
| Others |  emd_23694_additional_1.map.gz emd_23694_additional_1.map.gz emd_23694_additional_2.map.gz emd_23694_additional_2.map.gz | 152.1 MB 290.4 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-23694 http://ftp.pdbj.org/pub/emdb/structures/EMD-23694 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-23694 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-23694 | HTTPS FTP |
-Validation report
| Summary document |  emd_23694_validation.pdf.gz emd_23694_validation.pdf.gz | 567.5 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_23694_full_validation.pdf.gz emd_23694_full_validation.pdf.gz | 567 KB | Display | |
| Data in XML |  emd_23694_validation.xml.gz emd_23694_validation.xml.gz | 14.6 KB | Display | |
| Data in CIF |  emd_23694_validation.cif.gz emd_23694_validation.cif.gz | 19.8 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-23694 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-23694 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-23694 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-23694 | HTTPS FTP |
-Related structure data
| Related structure data |  7m6fMC  7m6dC  7m6eC  7m6gC  7m6hC  7m6iC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_23694.map.gz / Format: CCP4 / Size: 307.5 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_23694.map.gz / Format: CCP4 / Size: 307.5 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Sharpened map | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.869 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
-Additional map: Unsharpened map
| File | emd_23694_additional_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Unsharpened map | ||||||||||||
| Projections & Slices |
| ||||||||||||
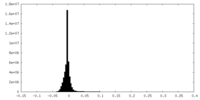
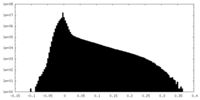
| Density Histograms |
-Additional map: Locally-refined map at Fab-RBD interface used for model building.
| File | emd_23694_additional_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Locally-refined map at Fab-RBD interface used for model building. | ||||||||||||
| Projections & Slices |
| ||||||||||||
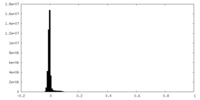
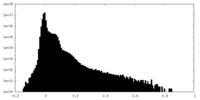
| Density Histograms |
- Sample components
Sample components
-Entire : Ternary complex of BG1-22 Fab bound to SARS-CoV-2 spike trimer
| Entire | Name: Ternary complex of BG1-22 Fab bound to SARS-CoV-2 spike trimer |
|---|---|
| Components |
|
-Supramolecule #1: Ternary complex of BG1-22 Fab bound to SARS-CoV-2 spike trimer
| Supramolecule | Name: Ternary complex of BG1-22 Fab bound to SARS-CoV-2 spike trimer type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1-#3 |
|---|---|
| Molecular weight | Theoretical: 720 KDa |
-Supramolecule #2: SARS-CoV-2 spike trimer
| Supramolecule | Name: SARS-CoV-2 spike trimer / type: complex / ID: 2 / Parent: 1 / Macromolecule list: #1 |
|---|---|
| Source (natural) | Organism:  |
-Supramolecule #3: BG1-22 Fab
| Supramolecule | Name: BG1-22 Fab / type: complex / ID: 3 / Parent: 1 / Macromolecule list: #2-#3 |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
-Macromolecule #1: Spike glycoprotein
| Macromolecule | Name: Spike glycoprotein / type: protein_or_peptide / ID: 1 Details: Pre-fusion stabilized HexaPro construct, including six proline substitutions and furin cleavage site RRAR 682-685 mutated to GSAS Number of copies: 3 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 142.427438 KDa |
| Recombinant expression | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: MFVFLVLLPL VSSQCVNLTT RTQLPPAYTN SFTRGVYYPD KVFRSSVLHS TQDLFLPFFS NVTWFHAIHV SGTNGTKRFD NPVLPFNDG VYFASTEKSN IIRGWIFGTT LDSKTQSLLI VNNATNVVIK VCEFQFCNDP FLGVYYHKNN KSWMESEFRV Y SSANNCTF ...String: MFVFLVLLPL VSSQCVNLTT RTQLPPAYTN SFTRGVYYPD KVFRSSVLHS TQDLFLPFFS NVTWFHAIHV SGTNGTKRFD NPVLPFNDG VYFASTEKSN IIRGWIFGTT LDSKTQSLLI VNNATNVVIK VCEFQFCNDP FLGVYYHKNN KSWMESEFRV Y SSANNCTF EYVSQPFLMD LEGKQGNFKN LREFVFKNID GYFKIYSKHT PINLVRDLPQ GFSALEPLVD LPIGINITRF QT LLALHRS YLTPGDSSSG WTAGAAAYYV GYLQPRTFLL KYNENGTITD AVDCALDPLS ETKCTLKSFT VEKGIYQTSN FRV QPTESI VRFPNITNLC PFGEVFNATR FASVYAWNRK RISNCVADYS VLYNSASFST FKCYGVSPTK LNDLCFTNVY ADSF VIRGD EVRQIAPGQT GKIADYNYKL PDDFTGCVIA WNSNNLDSKV GGNYNYLYRL FRKSNLKPFE RDISTEIYQA GSTPC NGVE GFNCYFPLQS YGFQPTNGVG YQPYRVVVLS FELLHAPATV CGPKKSTNLV KNKCVNFNFN GLTGTGVLTE SNKKFL PFQ QFGRDIADTT DAVRDPQTLE ILDITPCSFG GVSVITPGTN TSNQVAVLYQ DVNCTEVPVA IHADQLTPTW RVYSTGS NV FQTRAGCLIG AEHVNNSYEC DIPIGAGICA SYQTQTNSPG SASSVASQSI IAYTMSLGAE NSVAYSNNSI AIPTNFTI S VTTEILPVSM TKTSVDCTMY ICGDSTECSN LLLQYGSFCT QLNRALTGIA VEQDKNTQEV FAQVKQIYKT PPIKDFGGF NFSQILPDPS KPSKRSPIED LLFNKVTLAD AGFIKQYGDC LGDIAARDLI CAQKFNGLTV LPPLLTDEMI AQYTSALLAG TITSGWTFG AGPALQIPFP MQMAYRFNGI GVTQNVLYEN QKLIANQFNS AIGKIQDSLS STPSALGKLQ DVVNQNAQAL N TLVKQLSS NFGAISSVLN DILSRLDPPE AEVQIDRLIT GRLQSLQTYV TQQLIRAAEI RASANLAATK MSECVLGQSK RV DFCGKGY HLMSFPQSAP HGVVFLHVTY VPAQEKNFTT APAICHDGKA HFPREGVFVS NGTHWFVTQR NFYEPQIITT DNT FVSGNC DVVIGIVNNT VYDPLQPELD SFKEELDKYF KNHTSPDVDL GDISGINASV VNIQKEIDRL NEVAKNLNES LIDL QELGK YEQGSGYIPE APRDGQAYVR KDGEWVLLST FLGRSLEVLF QGPGHHHHHH HHSAWSHPQF EKGGGSGGGG SGGSA WSHP QFEK UniProtKB: Spike glycoprotein |
-Macromolecule #2: BG1-22 Fab Heavy Chain
| Macromolecule | Name: BG1-22 Fab Heavy Chain / type: protein_or_peptide / ID: 2 / Number of copies: 2 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 24.719713 KDa |
| Recombinant expression | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: EVQLVESGGG LVQPGGSLRL SCSVSGFTVS SNYMNWVRQA PGKGLEWVSV IYPGGSTFYP DSVKGRFTIS RDDSKNTLYL QMNSLRPED TAAYYCASSR PPIGQLVPGL DLDWFDPWGQ GTLVIVSSAS TKGPSVFPLA PSSKSTSGGT AALGCLVKDY F PEPVTVSW ...String: EVQLVESGGG LVQPGGSLRL SCSVSGFTVS SNYMNWVRQA PGKGLEWVSV IYPGGSTFYP DSVKGRFTIS RDDSKNTLYL QMNSLRPED TAAYYCASSR PPIGQLVPGL DLDWFDPWGQ GTLVIVSSAS TKGPSVFPLA PSSKSTSGGT AALGCLVKDY F PEPVTVSW NSGALTSGVH TFPAVLQSSG LYSLSSVVTV PSSSLGTQTY ICNVNHKPSN TKVDKRVEPK SCDKT |
-Macromolecule #3: BG1-22 Fab Light Chain
| Macromolecule | Name: BG1-22 Fab Light Chain / type: protein_or_peptide / ID: 3 / Number of copies: 2 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 22.944305 KDa |
| Recombinant expression | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: QSVLTQPPSV SGAPGQRVTI SCTGSSSNIG AGYYVHWYQQ HPGTAPKLLI YGNSNRPSGV PDRFSGSKSG TSASLAITGL QAEDEADYY CQSYDSRLSG WVFGGGTKLT VLGQPKAAPS VTLFPPSSEE LQANKATLVC LISDFYPGAV TVAWKADSSP V KAGVETTT ...String: QSVLTQPPSV SGAPGQRVTI SCTGSSSNIG AGYYVHWYQQ HPGTAPKLLI YGNSNRPSGV PDRFSGSKSG TSASLAITGL QAEDEADYY CQSYDSRLSG WVFGGGTKLT VLGQPKAAPS VTLFPPSSEE LQANKATLVC LISDFYPGAV TVAWKADSSP V KAGVETTT PSKQSNNKYA ASSYLSLTPE QWKSHRSYSC QVTHEGSTVE KTVAPTECS |
-Macromolecule #5: 2-acetamido-2-deoxy-beta-D-glucopyranose
| Macromolecule | Name: 2-acetamido-2-deoxy-beta-D-glucopyranose / type: ligand / ID: 5 / Number of copies: 33 / Formula: NAG |
|---|---|
| Molecular weight | Theoretical: 221.208 Da |
| Chemical component information |  ChemComp-NAG: |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 3.0 mg/mL | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Buffer | pH: 8 Component:
| |||||||||
| Grid | Model: UltrAuFoil R1.2/1.3 / Material: GOLD / Mesh: 300 / Support film - Material: GOLD / Support film - topology: HOLEY / Pretreatment - Type: GLOW DISCHARGE / Pretreatment - Time: 60 sec. / Pretreatment - Atmosphere: AIR | |||||||||
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 295 K / Instrument: FEI VITROBOT MARK IV / Details: 3s blot, 0 blot force. | |||||||||
| Details | Monodisperse sample |
- Electron microscopy
Electron microscopy
| Microscope | FEI TECNAI ARCTICA |
|---|---|
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Number grids imaged: 1 / Number real images: 3386 / Average exposure time: 3.6 sec. / Average electron dose: 60.0 e/Å2 |
| Electron beam | Acceleration voltage: 200 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | C2 aperture diameter: 50.0 µm / Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm / Nominal defocus max: 2.0 µm / Nominal defocus min: 0.7000000000000001 µm / Nominal magnification: 45000 |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Talos Arctica / Image courtesy: FEI Company |
+ Image processing
Image processing
-Atomic model buiding 1
| Initial model | PDB ID: Chain - Source name: PDB / Chain - Initial model type: experimental model |
|---|---|
| Refinement | Space: REAL / Protocol: RIGID BODY FIT / Target criteria: Correlation coefficient |
| Output model |  PDB-7m6f: |
 Movie
Movie Controller
Controller


















 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)