[English] 日本語
 Yorodumi
Yorodumi- EMDB-23626: cryo-EM Structure of Nucleosome containing mouse histone variant H2A.Z -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-23626 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | cryo-EM Structure of Nucleosome containing mouse histone variant H2A.Z | |||||||||
 Map data Map data | cryo-EM map of nucleosome containing histone variant H2A.Z | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | chromatin / nucleosome / histone variant / epigenetics / transcription / DNA BINDING PROTEIN / DNA BINDING PROTEIN-DNA complex | |||||||||
| Function / homology |  Function and homology information Function and homology informationnucleosomal DNA binding / RNA polymerase II core promoter sequence-specific DNA binding / heterochromatin / cellular response to estradiol stimulus / euchromatin / structural constituent of chromatin / nucleosome / nucleosome assembly / chromatin organization / RNA polymerase II cis-regulatory region sequence-specific DNA binding ...nucleosomal DNA binding / RNA polymerase II core promoter sequence-specific DNA binding / heterochromatin / cellular response to estradiol stimulus / euchromatin / structural constituent of chromatin / nucleosome / nucleosome assembly / chromatin organization / RNA polymerase II cis-regulatory region sequence-specific DNA binding / protein heterodimerization activity / positive regulation of transcription by RNA polymerase II / DNA binding / nucleus Similarity search - Function | |||||||||
| Biological species |  | |||||||||
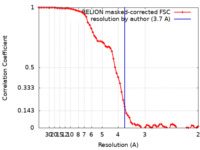
| Method | single particle reconstruction / cryo EM / Resolution: 3.7 Å | |||||||||
 Authors Authors | Tan D / Lewis T | |||||||||
| Funding support |  United States, 2 items United States, 2 items
| |||||||||
 Citation Citation |  Journal: Nucleic Acids Res / Year: 2021 Journal: Nucleic Acids Res / Year: 2021Title: Structural basis of chromatin regulation by histone variant H2A.Z. Authors: Tyler S Lewis / Vladyslava Sokolova / Harry Jung / Honkit Ng / Dongyan Tan /  Abstract: The importance of histone variant H2A.Z in transcription regulation has been well established, yet its mechanism-of-action remains enigmatic. Conflicting evidence exists in support of both an ...The importance of histone variant H2A.Z in transcription regulation has been well established, yet its mechanism-of-action remains enigmatic. Conflicting evidence exists in support of both an activating and a repressive role of H2A.Z in transcription. Here we report cryo-electron microscopy (cryo-EM) structures of nucleosomes and chromatin fibers containing H2A.Z and those containing canonical H2A. The structures show that H2A.Z incorporation results in substantial structural changes in both nucleosome and chromatin fiber. While H2A.Z increases the mobility of DNA terminus in nucleosomes, it simultaneously enables nucleosome arrays to form a more regular and condensed chromatin fiber. We also demonstrated that H2A.Z's ability to enhance nucleosomal DNA mobility is largely attributed to its characteristic shorter C-terminus. Our study provides the structural basis for H2A.Z-mediated chromatin regulation, showing that the increase flexibility of the DNA termini in H2A.Z nucleosomes is central to its dual-functions in chromatin regulation and in transcription. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_23626.map.gz emd_23626.map.gz | 26.9 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-23626-v30.xml emd-23626-v30.xml emd-23626.xml emd-23626.xml | 26.7 KB 26.7 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_23626_fsc.xml emd_23626_fsc.xml | 6.8 KB | Display |  FSC data file FSC data file |
| Images |  emd_23626.png emd_23626.png | 199.4 KB | ||
| Filedesc metadata |  emd-23626.cif.gz emd-23626.cif.gz | 6.8 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-23626 http://ftp.pdbj.org/pub/emdb/structures/EMD-23626 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-23626 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-23626 | HTTPS FTP |
-Related structure data
| Related structure data |  7m1xMC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_23626.map.gz / Format: CCP4 / Size: 30.5 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_23626.map.gz / Format: CCP4 / Size: 30.5 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | cryo-EM map of nucleosome containing histone variant H2A.Z | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.1 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
- Sample components
Sample components
+Entire : Nucleosome core particle containing variant H2A.Z and canonical c...
+Supramolecule #1: Nucleosome core particle containing variant H2A.Z and canonical c...
+Supramolecule #2: Histone H3
+Supramolecule #3: Histone H4
+Supramolecule #4: Histone variant H2A.Z
+Supramolecule #5: Histone H2B
+Supramolecule #6: DNA (136-MER)
+Supramolecule #7: DNA (136-MER)
+Macromolecule #1: DNA (136-MER)
+Macromolecule #2: DNA (136-MER)
+Macromolecule #3: Histone H3
+Macromolecule #4: Histone H4
+Macromolecule #5: Histone H2A.Z
+Macromolecule #6: Histone H2B 1.1
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 1 mg/mL | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Buffer | pH: 8 Component:
| |||||||||||||||
| Grid | Model: Quantifoil R1.2/1.3 / Material: COPPER / Mesh: 400 / Support film - Material: CARBON / Support film - topology: HOLEY / Pretreatment - Type: GLOW DISCHARGE / Pretreatment - Time: 30 sec. / Pretreatment - Atmosphere: AIR | |||||||||||||||
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 277.15 K / Instrument: FEI VITROBOT MARK IV / Details: blot for 4.5 seconds before plunging. | |||||||||||||||
| Details | This sample was mono-disperse. |
- Electron microscopy
Electron microscopy
| Microscope | FEI TALOS ARCTICA |
|---|---|
| Image recording | Film or detector model: FEI FALCON III (4k x 4k) / Detector mode: COUNTING / Number grids imaged: 3 / Number real images: 1950 / Average exposure time: 60.0 sec. / Average electron dose: 33.0 e/Å2 |
| Electron beam | Acceleration voltage: 200 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | C2 aperture diameter: 50.0 µm / Calibrated magnification: 123811 / Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm / Nominal defocus max: 2.5 µm / Nominal defocus min: 0.8 µm / Nominal magnification: 92000 |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Talos Arctica / Image courtesy: FEI Company |
+ Image processing
Image processing
-Atomic model buiding 1
| Initial model |
| ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Space: REAL / Protocol: FLEXIBLE FIT / Target criteria: rmsd | ||||||||||||||||||||||
| Output model |  PDB-7m1x: |
 Movie
Movie Controller
Controller

















 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)