+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-23517 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Nse5-6 complex | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | SMC5/6 / Nse5-6 / Nse5 / Nse6 / complex / SUMO-binding / STRUCTURAL PROTEIN | |||||||||
| Function / homology |  Function and homology information Function and homology informationSmc5-Smc6 complex / SUMO is conjugated to E1 (UBA2:SAE1) / SUMOylation of nuclear envelope proteins / SUMO is transferred from E1 to E2 (UBE2I, UBC9) / SUMO is proteolytically processed / SUMOylation of transcription factors / Postmitotic nuclear pore complex (NPC) reformation / SUMOylation of transcription cofactors / septin ring / SUMOylation of DNA damage response and repair proteins ...Smc5-Smc6 complex / SUMO is conjugated to E1 (UBA2:SAE1) / SUMOylation of nuclear envelope proteins / SUMO is transferred from E1 to E2 (UBE2I, UBC9) / SUMO is proteolytically processed / SUMOylation of transcription factors / Postmitotic nuclear pore complex (NPC) reformation / SUMOylation of transcription cofactors / septin ring / SUMOylation of DNA damage response and repair proteins / Transcriptional and post-translational regulation of MITF-M expression and activity / SUMOylation of DNA replication proteins / ATPase inhibitor activity / SUMOylation of SUMOylation proteins / Recruitment and ATM-mediated phosphorylation of repair and signaling proteins at DNA double strand breaks / chromatin looping / SUMOylation of RNA binding proteins / SUMOylation of chromatin organization proteins / regulation of telomere maintenance / ubiquitin-like protein ligase binding / protein sumoylation / condensed nuclear chromosome / double-strand break repair via homologous recombination / protein tag activity / chromosome, telomeric region / DNA repair / identical protein binding / nucleus / cytoplasm Similarity search - Function | |||||||||
| Biological species |  | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 3.2 Å | |||||||||
 Authors Authors | Yu Y / Li SB | |||||||||
| Funding support |  United States, 1 items United States, 1 items
| |||||||||
 Citation Citation |  Journal: Proc Natl Acad Sci U S A / Year: 2021 Journal: Proc Natl Acad Sci U S A / Year: 2021Title: Integrative analysis reveals unique structural and functional features of the Smc5/6 complex. Authors: You Yu / Shibai Li / Zheng Ser / Tanmoy Sanyal / Koyi Choi / Bingbing Wan / Huihui Kuang / Andrej Sali / Alex Kentsis / Dinshaw J Patel / Xiaolan Zhao /  Abstract: Structural maintenance of chromosomes (SMC) complexes are critical chromatin modulators. In eukaryotes, the cohesin and condensin SMC complexes organize chromatin, while the Smc5/6 complex directly ...Structural maintenance of chromosomes (SMC) complexes are critical chromatin modulators. In eukaryotes, the cohesin and condensin SMC complexes organize chromatin, while the Smc5/6 complex directly regulates DNA replication and repair. The molecular basis for the distinct functions of Smc5/6 is poorly understood. Here, we report an integrative structural study of the budding yeast Smc5/6 holo-complex using electron microscopy, cross-linking mass spectrometry, and computational modeling. We show that the Smc5/6 complex possesses several unique features, while sharing some architectural characteristics with other SMC complexes. In contrast to arm-folded structures of cohesin and condensin, Smc5 and Smc6 arm regions do not fold back on themselves. Instead, these long filamentous regions interact with subunits uniquely acquired by the Smc5/6 complex, namely the Nse2 SUMO ligase and the Nse5/Nse6 subcomplex, with the latter also serving as a linchpin connecting distal parts of the complex. Our 3.0-Å resolution cryoelectron microscopy structure of the Nse5/Nse6 core further reveals a clasped-hand topology and a dimeric interface important for cell growth. Finally, we provide evidence that Nse5/Nse6 uses its SUMO-binding motifs to contribute to Nse2-mediated sumoylation. Collectively, our integrative study identifies distinct structural features of the Smc5/6 complex and functional cooperation among its coevolved unique subunits. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_23517.map.gz emd_23517.map.gz | 78.5 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-23517-v30.xml emd-23517-v30.xml emd-23517.xml emd-23517.xml | 14.3 KB 14.3 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_23517.png emd_23517.png | 163.8 KB | ||
| Filedesc metadata |  emd-23517.cif.gz emd-23517.cif.gz | 6.5 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-23517 http://ftp.pdbj.org/pub/emdb/structures/EMD-23517 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-23517 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-23517 | HTTPS FTP |
-Related structure data
| Related structure data |  7ltoMC  7sdeM M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_23517.map.gz / Format: CCP4 / Size: 83.7 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_23517.map.gz / Format: CCP4 / Size: 83.7 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.064 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
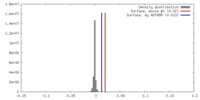
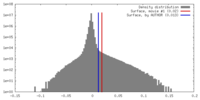
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
- Sample components
Sample components
-Entire : Nse5-Nse6 complex
| Entire | Name: Nse5-Nse6 complex |
|---|---|
| Components |
|
-Supramolecule #1: Nse5-Nse6 complex
| Supramolecule | Name: Nse5-Nse6 complex / type: complex / ID: 1 / Parent: 0 / Macromolecule list: all |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 133 KDa |
-Macromolecule #1: Non-structural maintenance of chromosome element 5
| Macromolecule | Name: Non-structural maintenance of chromosome element 5 / type: protein_or_peptide / ID: 1 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 65.276961 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MADLNWISAG HMDGALINSV LYVSPRNGAH YFVELTEKHL LAFEMLNSMC LLENYDHVLL FLECQFGKSH NLAVIPFDII LVLFTLSTL SEYYKEPILR ANDPYNTSRE TLSRRALKLL QKYLAILKEF DSEQYNLYDL ELLRCQFFLA IDTLTPKKQK W GFDRFRRT ...String: MADLNWISAG HMDGALINSV LYVSPRNGAH YFVELTEKHL LAFEMLNSMC LLENYDHVLL FLECQFGKSH NLAVIPFDII LVLFTLSTL SEYYKEPILR ANDPYNTSRE TLSRRALKLL QKYLAILKEF DSEQYNLYDL ELLRCQFFLA IDTLTPKKQK W GFDRFRRT KSESGVTYRQ NASVDPELDQ AKTFKNPYRS YISCLEQRNT ILGNRLLNLK LNEPGEFINM ILWTLSNSLQ ES TPLFLSS HEIWMPLLEI LIDLFSCRQD YFIQHEVAQN VSKSLFVQRL SESPLAVFFE SLNTRNFANR FSEYVFLNCD YKL PSDNYA TPVHPVYNGE NTIVDTYIPT IKCSPLYKSQ KSLALRRKLI GSCFKLLLRV PDGHRLITPR IVADDVIQGI SRTL ASFND ILQFKKFFMT ENLSQESYFI PLLAEGTLSE ILKDTQECVV ILTLVENLSD GVSFCNEVIG LVKSKCFAFT EQCSQ ASYE EAVLNIEKCD VCLLVLLRYL LHLIGTEAIL DAKEQLEMLH AIEKNDSGRR QWAKALNLGN DPPLLYPIVS QMFGVH DKS VIIE UniProtKB: Non-structural maintenance of chromosome element 5 |
-Macromolecule #2: Ubiquitin-like protein SMT3,DNA repair protein KRE29 chimera
| Macromolecule | Name: Ubiquitin-like protein SMT3,DNA repair protein KRE29 chimera type: protein_or_peptide / ID: 2 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 67.64518 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MGSSHHHHHH SSGLVPRGSH MASMSDSEVN QEAKPEVKPE VKPETHINLK VSDGSSEIFF KIKKTTPLRR LMEAFAKRQG KEMDSLRFL YDGIRIQADQ TPEDLDMEDN DIIEAHREQI GGSMGSVNSS PNEEFETVPD SQISGFDSPL IPTSVGSYFR D DDDDEKVH ...String: MGSSHHHHHH SSGLVPRGSH MASMSDSEVN QEAKPEVKPE VKPETHINLK VSDGSSEIFF KIKKTTPLRR LMEAFAKRQG KEMDSLRFL YDGIRIQADQ TPEDLDMEDN DIIEAHREQI GGSMGSVNSS PNEEFETVPD SQISGFDSPL IPTSVGSYFR D DDDDEKVH PNFISDPEND SLNSDEEFSS LENSDLNLSG AKAESGDDFD PILKRTIISK RKAPSNNEDE EIVKTPRKLV NY VPLKIFN LGDSFDDTIT TTVAKLQDLK KEILDSPRSN KSIVITSNTV AKSELQKSIK FSGSIPEIYL DVVTKETISD KYK DWHFIS KNCHYEQLMD LEMKDTAYSF LFGSSRSQGK VPEFVHLKCP SITNLLVLFG VNQEKCNSLK INYEKKENSR YDNL CTIFP VNKMLKFLMY FYSDDDNDDV REFFLKAFIC LILDRKVFNA MESDHRLCFK VLELFNEAHF INSYFEIVDK NDFFL HYRL LQIFPHLQSA LLRRRFSEKQ GRTETIQQNI IKEFNEFFDC KNYKNLLYFI LTMYGSKFIP FGPKCQVTEY FKDCIL DIS NETTNDVEIS ILKGILNLFS KIR UniProtKB: Ubiquitin-like protein SMT3, DNA repair protein KRE29 |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 0.3 mg/mL |
|---|---|
| Buffer | pH: 7.5 |
| Grid | Model: UltrAuFoil R1.2/1.3 / Material: GOLD / Mesh: 300 / Pretreatment - Type: GLOW DISCHARGE / Pretreatment - Time: 120 sec. |
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Average electron dose: 53.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Calibrated magnification: 47262 / Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal magnification: 22500 |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller

















 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)