[English] 日本語
 Yorodumi
Yorodumi- EMDB-22867: Cryo-EM Structures of AdeB from Acinetobacter baumannii: AdeB-II -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-22867 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo-EM Structures of AdeB from Acinetobacter baumannii: AdeB-II | |||||||||
 Map data Map data | Density modified map of AdeB-II | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | AdeB / Acinetobacter baumannii / Membrane Protein / Cryo-EM / RND transporter / TRANSPORT PROTEIN | |||||||||
| Function / homology | Hydrophobe/amphiphile efflux-1 HAE1 / Multidrug efflux transporter AcrB TolC docking domain, DN/DC subdomains / Acriflavin resistance protein / AcrB/AcrD/AcrF family / efflux transmembrane transporter activity / xenobiotic transmembrane transporter activity / response to toxic substance / plasma membrane / Efflux pump membrane transporter Function and homology information Function and homology information | |||||||||
| Biological species |  Acinetobacter baumannii (bacteria) Acinetobacter baumannii (bacteria) | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 3.21 Å | |||||||||
 Authors Authors | Morgan CE / Yu EW | |||||||||
| Funding support |  United States, 1 items United States, 1 items
| |||||||||
 Citation Citation |  Journal: mBio / Year: 2021 Journal: mBio / Year: 2021Title: Cryoelectron Microscopy Structures of AdeB Illuminate Mechanisms of Simultaneous Binding and Exporting of Substrates. Authors: Christopher E Morgan / Przemyslaw Glaza / Inga V Leus / Anhthu Trinh / Chih-Chia Su / Meng Cui / Helen I Zgurskaya / Edward W Yu /  Abstract: is a Gram-negative pathogen that has emerged as one of the most highly antibiotic-resistant bacteria worldwide. Multidrug efflux within these highly drug-resistant strains and other opportunistic ... is a Gram-negative pathogen that has emerged as one of the most highly antibiotic-resistant bacteria worldwide. Multidrug efflux within these highly drug-resistant strains and other opportunistic pathogens is a major cause of failure of drug-based treatments of infectious diseases. The best-characterized multidrug efflux system in is the prevalent rug fflux B (AdeB) pump, which is a member of the resistance-nodulation-cell division (RND) superfamily. Here, we report six structures of the trimeric AdeB multidrug efflux pump in the presence of ethidium bromide using single-particle cryoelectron microscopy (cryo-EM). These structures allow us to directly observe various novel conformational states of the AdeB trimer, including the transmembrane region of trimeric AdeB can be associated with form a trimer assembly or dissociated into "dimer plus monomer" and "monomer plus monomer plus monomer" configurations. We also discover that a single AdeB protomer can simultaneously anchor a number of ethidium ligands and that different AdeB protomers can bind ethidium molecules simultaneously. Combined with molecular dynamics (MD) simulations, we reveal a drug transport mechanism that involves multiple multidrug-binding sites and various transient states of the AdeB membrane protein. Our data suggest that each AdeB protomer within the trimer binds and exports drugs independently. has emerged as one of the most highly antibiotic-resistant Gram-negative pathogens. The prevalent AdeB multidrug efflux pump mediates resistance to a broad spectrum of clinically relevant antimicrobial agents. Here, we report six cryo-EM structures of the trimeric AdeB pump in the presence of ethidium bromide. We discover that a single AdeB protomer can simultaneously anchor a number of ligands, and different AdeB protomers can bind ethidium molecules simultaneously. The results indicate that each AdeB protomer within the trimer recognizes and extrudes drugs independently. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_22867.map.gz emd_22867.map.gz | 8 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-22867-v30.xml emd-22867-v30.xml emd-22867.xml emd-22867.xml | 10.4 KB 10.4 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_22867.png emd_22867.png | 102.3 KB | ||
| Filedesc metadata |  emd-22867.cif.gz emd-22867.cif.gz | 5.7 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-22867 http://ftp.pdbj.org/pub/emdb/structures/EMD-22867 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-22867 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-22867 | HTTPS FTP |
-Related structure data
| Related structure data |  7kgeMC  7kgdC  7kgfC  7kggC  7kghC  7kgiC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_22867.map.gz / Format: CCP4 / Size: 8.6 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_22867.map.gz / Format: CCP4 / Size: 8.6 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
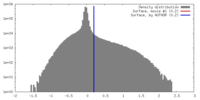
| Annotation | Density modified map of AdeB-II | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. generated in cubic-lattice coordinate | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.08 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
- Sample components
Sample components
-Entire : AdeB
| Entire | Name: AdeB |
|---|---|
| Components |
|
-Supramolecule #1: AdeB
| Supramolecule | Name: AdeB / type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1 |
|---|---|
| Source (natural) | Organism:  Acinetobacter baumannii (bacteria) Acinetobacter baumannii (bacteria) |
-Macromolecule #1: Efflux pump membrane transporter
| Macromolecule | Name: Efflux pump membrane transporter / type: protein_or_peptide / ID: 1 / Number of copies: 3 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Acinetobacter baumannii (bacteria) Acinetobacter baumannii (bacteria) |
| Molecular weight | Theoretical: 112.588297 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MSQFFIRRPV FAWVIAIFII IFGLLSIPKL PIARFPSVAP PQVNISATYP GATAKTINDS VVTLIERELS GVKNLLYYSA TTDTSGTAE ITATFKPGTD VEMAQVDVQN KIKAVEARLP QVVRQQGLQV EASSSGFLML VGINSPNNQY SEVDLSDYLV R NVVEELKR ...String: MSQFFIRRPV FAWVIAIFII IFGLLSIPKL PIARFPSVAP PQVNISATYP GATAKTINDS VVTLIERELS GVKNLLYYSA TTDTSGTAE ITATFKPGTD VEMAQVDVQN KIKAVEARLP QVVRQQGLQV EASSSGFLML VGINSPNNQY SEVDLSDYLV R NVVEELKR VEGVGKVQSF GAEKAMRIWV DPNKLVSYGL SISDVNNAIR ENNVEIAPGR LGDLPAEKGQ LITIPLSAQG QL SSLEQFK NISLKSKTNG SVIKLSDVAN VEIGSQAYNF AILENGKPAT AAAIQLSPGA NAVKTAEGVR AKIEELKLNL PEG MEFSIP YDTAPFVKIS IEKVIHTLLE AMVLVFIVMY LFLHNVRYTL IPAIVAPIAL LGTFTVMLLA GFSINVLTMF GMVL AIGII VDDAIVVVEN VERIMATEGL SPKDATSKAM KEITSPIIGI TLVLAAVFLP MAFASGSVGV IYKQFTLTMS VSILF SALL ALILTPALCA TILKPIDGHH QKKGFFAWFD RSFDKVTKKY ELMLLKIIKH TVPMMVIFLV ITGITFAGMK YWPTAF MPE EDQGWFMTSF QLPSDATAER TRNVVNQFEN NLKDNPDVKS NTAILGWGFS GAGQNVAVAF TTLKDFKERT SSASKMT SD VNSSMANSTE GETMAVLPPA IDELGTFSGF SLRLQDRANL GMPALLAAQD ELMAMAAKNK KFYMVWNEGL PQGDNISL K IDREKLSALG VKFSDVSDII STSMGSMYIN DFPNQGRMQQ VIVQVEAKSR MQLKDILNLK VMGSSGQLVS LSEVVTPQW NKAPQQYNRY NGRPSLSIAG IPNFDTSSGE AMREMEQLIA KLPKGIGYEW TGISLQEKQS ESQMAFLLGL SMLVVFLVLA ALYESWAIP LSVMLVVPLG IFGAIIAIMS RGLMNDVFFK IGLITIIGLS AKNAILIVEF AKMLKEEGMS LIEATVAAAK L RLRPILMT SLAFTCGVIP LVIATGASSE TQHALGTGVF GGMISATILA IFFVPVFFIF ILGAVEKLFS SKKKISS UniProtKB: Efflux pump membrane transporter |
-Macromolecule #2: PHOSPHATIDYLETHANOLAMINE
| Macromolecule | Name: PHOSPHATIDYLETHANOLAMINE / type: ligand / ID: 2 / Number of copies: 3 / Formula: PTY |
|---|---|
| Molecular weight | Theoretical: 734.039 Da |
| Chemical component information |  ChemComp-PTY: |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 6.5 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Detector mode: SUPER-RESOLUTION / Average electron dose: 40.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: SPOT SCAN / Imaging mode: BRIGHT FIELD |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
- Image processing
Image processing
| Startup model | Type of model: NONE |
|---|---|
| Final reconstruction | Applied symmetry - Point group: C1 (asymmetric) / Resolution.type: BY AUTHOR / Resolution: 3.21 Å / Resolution method: FSC 0.143 CUT-OFF / Software - Name: cryoSPARC (ver. 2.15) / Number images used: 95552 |
| Initial angle assignment | Type: MAXIMUM LIKELIHOOD |
| Final angle assignment | Type: MAXIMUM LIKELIHOOD |
 Movie
Movie Controller
Controller















 X (Sec.)
X (Sec.) Y (Row.)
Y (Row.) Z (Col.)
Z (Col.)