[English] 日本語
 Yorodumi
Yorodumi- EMDB-22489: Cryo-EM structure of Grafix-stabilized PaFS prenyltransferase oct... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-22489 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo-EM structure of Grafix-stabilized PaFS prenyltransferase octamer with cyclase domain capping octameric central pore | |||||||||
 Map data Map data | Prenyltransferase octamer with capping cyclase domain | |||||||||
 Sample Sample |
| |||||||||
| Function / homology |  Function and homology information Function and homology informationfusicocca-2,10(14)-diene synthase / alcohol biosynthetic process / mycotoxin biosynthetic process / geranylgeranyl diphosphate synthase / geranylgeranyl diphosphate synthase activity / isoprenoid biosynthetic process / lyase activity / metal ion binding Similarity search - Function | |||||||||
| Biological species |  Diaporthe amygdali (fungus) Diaporthe amygdali (fungus) | |||||||||
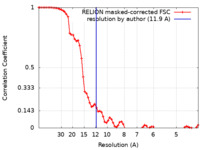
| Method | single particle reconstruction / cryo EM / Resolution: 11.9 Å | |||||||||
 Authors Authors | Faylo JL / van Eeuwen T / Murakami K / Christianson DW | |||||||||
| Funding support |  United States, 1 items United States, 1 items
| |||||||||
 Citation Citation |  Journal: Nat Commun / Year: 2021 Journal: Nat Commun / Year: 2021Title: Structural insight on assembly-line catalysis in terpene biosynthesis. Authors: Jacque L Faylo / Trevor van Eeuwen / Hee Jong Kim / Jose J Gorbea Colón / Benjamin A Garcia / Kenji Murakami / David W Christianson /  Abstract: Fusicoccadiene synthase from Phomopsis amygdali (PaFS) is a unique bifunctional terpenoid synthase that catalyzes the first two steps in the biosynthesis of the diterpene glycoside Fusicoccin A, a ...Fusicoccadiene synthase from Phomopsis amygdali (PaFS) is a unique bifunctional terpenoid synthase that catalyzes the first two steps in the biosynthesis of the diterpene glycoside Fusicoccin A, a mediator of 14-3-3 protein interactions. The prenyltransferase domain of PaFS generates geranylgeranyl diphosphate, which the cyclase domain then utilizes to generate fusicoccadiene, the tricyclic hydrocarbon skeleton of Fusicoccin A. Here, we use cryo-electron microscopy to show that the structure of full-length PaFS consists of a central octameric core of prenyltransferase domains, with the eight cyclase domains radiating outward via flexible linker segments in variable splayed-out positions. Cryo-electron microscopy and chemical crosslinking experiments additionally show that compact conformations can be achieved in which cyclase domains are more closely associated with the prenyltransferase core. This structural analysis provides a framework for understanding substrate channeling, since most of the geranylgeranyl diphosphate generated by the prenyltransferase domains remains on the enzyme for cyclization to form fusicoccadiene. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_22489.map.gz emd_22489.map.gz | 20.6 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-22489-v30.xml emd-22489-v30.xml emd-22489.xml emd-22489.xml | 20 KB 20 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_22489_fsc.xml emd_22489_fsc.xml | 7 KB | Display |  FSC data file FSC data file |
| Images |  emd_22489.png emd_22489.png | 63.6 KB | ||
| Masks |  emd_22489_msk_1.map emd_22489_msk_1.map emd_22489_msk_2.map emd_22489_msk_2.map | 27 MB 27 MB |  Mask map Mask map | |
| Others |  emd_22489_additional_1.map.gz emd_22489_additional_1.map.gz emd_22489_half_map_1.map.gz emd_22489_half_map_1.map.gz emd_22489_half_map_2.map.gz emd_22489_half_map_2.map.gz | 1.8 MB 20.6 MB 20.7 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-22489 http://ftp.pdbj.org/pub/emdb/structures/EMD-22489 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-22489 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-22489 | HTTPS FTP |
-Related structure data
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_22489.map.gz / Format: CCP4 / Size: 27 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_22489.map.gz / Format: CCP4 / Size: 27 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Prenyltransferase octamer with capping cyclase domain | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 2.16 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
-Mask #1
| File |  emd_22489_msk_1.map emd_22489_msk_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
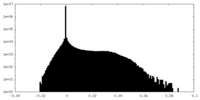
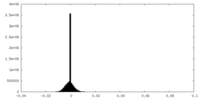
| Density Histograms |
-Mask #2
| File |  emd_22489_msk_2.map emd_22489_msk_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Additional map: Processed map of prenyltransferase octamer with capping cyclase...
| File | emd_22489_additional_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Processed map of prenyltransferase octamer with capping cyclase domain | ||||||||||||
| Projections & Slices |
| ||||||||||||
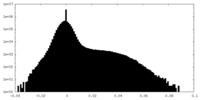
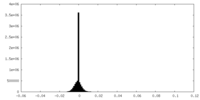
| Density Histograms |
-Half map: #1
| File | emd_22489_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: #2
| File | emd_22489_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : Phomopsis amygdali fusicoccadiene synthase (PaFS)
| Entire | Name: Phomopsis amygdali fusicoccadiene synthase (PaFS) |
|---|---|
| Components |
|
-Supramolecule #1: Phomopsis amygdali fusicoccadiene synthase (PaFS)
| Supramolecule | Name: Phomopsis amygdali fusicoccadiene synthase (PaFS) / type: complex / ID: 1 / Parent: 0 / Macromolecule list: all Details: PaFS octameric prenyltransferase domain is resolved with density corresonding to a single cyclase domain on top of the octamer. Molecular weight reflects the calculated molecular weight of a ...Details: PaFS octameric prenyltransferase domain is resolved with density corresonding to a single cyclase domain on top of the octamer. Molecular weight reflects the calculated molecular weight of a prenyltransferase octamer plus one cyclase domain. |
|---|---|
| Source (natural) | Organism:  Diaporthe amygdali (fungus) Diaporthe amygdali (fungus) |
| Recombinant expression | Organism:  |
| Molecular weight | Theoretical: 430 KDa |
-Macromolecule #1: Fusicoccadiene synthase
| Macromolecule | Name: Fusicoccadiene synthase / type: protein_or_peptide / ID: 1 Details: The final reconstruction accounts for an octamer of the C-terminal transferase domain, residues 414-719. Density on top of the octamer accounts for the N-terminal cyclase domain Enantiomer: LEVO / EC number: fusicocca-2,10(14)-diene synthase |
|---|---|
| Source (natural) | Organism:  Diaporthe amygdali (fungus) Diaporthe amygdali (fungus) |
| Recombinant expression | Organism:  |
| Sequence | String: MGSSHHHHHH SSGLVPRGSH MEFKYSEVVE PSTYYTEGLC EGIDVRKSKF TTLEDRGAIR AHEDWNKHIG PCREYRGTLG PRFSFISVAV PECIPERLEV ISYANEFAFL HDDVTDHVGH DTGEVENDEM MTVFLEAAHT GAIDTSNKVD IRRAGKKRIQ SQLFLEMLAI ...String: MGSSHHHHHH SSGLVPRGSH MEFKYSEVVE PSTYYTEGLC EGIDVRKSKF TTLEDRGAIR AHEDWNKHIG PCREYRGTLG PRFSFISVAV PECIPERLEV ISYANEFAFL HDDVTDHVGH DTGEVENDEM MTVFLEAAHT GAIDTSNKVD IRRAGKKRIQ SQLFLEMLAI DPECAKTTMK SWARFVEVGS SRQHETRFVE LAKYIPYRIM DVGEMFWFGL VTFGLGLHIP DHELELCREL MANAWIAVGL QNDIWSWPKE RDAATLHGKD HVVNAIWVLM QEHQTDVDGA MQICRKLIVE YVAKYLEVIE ATKNDESISL DLRKYLDAML YSISGNVVWS LECPRYNPDV SFNKTQLEWM RQGLPSLESC PVLARSPEID SDESAVSPTA DESDSTEDSL GSGSRQDSSL STGLSLSPVH SNEGKDLQRV DTDHIFFEKA VLEAPYDYIA SMPSKGVRDQ FIDALNDWLR VPDVKVGKIK DAVRVLHNSS LLLDDFQDNS PLRRGKPSTH NIFGSAQTVN TATYSIIKAI GQIMEFSAGE SVQEVMNSIM ILFQGQAMDL FWTYNGHVPS EEEYYRMIDQ KTGQLFSIAT SLLLNAADNE IPRTKIQSCL HRLTRLLGRC FQICDDYQNL VSADYTKQKG FCEDLDEGKW SLALIHMIHK QRSHMALLNV LSTGRKHGGM TLEQKQFVLD IIEEEKSLDY TRSVMMDLHV QLRAEIGRIE ILLDSPNPAM RLLLELLRV |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 0.3 mg/mL |
|---|---|
| Buffer | pH: 7.5 |
| Grid | Details: unspecified |
| Vitrification | Cryogen name: ETHANE / Instrument: LEICA EM CPC / Details: Blot for 2.5 seconds before plunging. |
| Details | Protein was GraFix-stabilized prior to vitrification. |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Number grids imaged: 1 / Number real images: 6544 / Average electron dose: 50.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: SPOT SCAN / Imaging mode: BRIGHT FIELD |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
+ Image processing
Image processing
-Atomic model buiding 1
| Refinement | Protocol: AB INITIO MODEL |
|---|
 Movie
Movie Controller
Controller



















 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)