[English] 日本語
 Yorodumi
Yorodumi- EMDB-22478: Cryo-EM map of the human BAF-nucleosome complex refined with a ma... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-22478 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo-EM map of the human BAF-nucleosome complex refined with a mask for the nucleosome and the ATPase-ARP modules | |||||||||
 Map data Map data | Cryo-EM map of the human BAF-nucleosome complex refined with a mask for the nucleosome and the ATPase-ARP modules | |||||||||
 Sample Sample |
| |||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | |||||||||
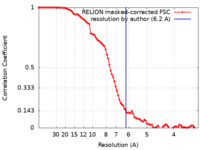
| Method | single particle reconstruction / cryo EM / Resolution: 6.2 Å | |||||||||
 Authors Authors | Suzuki H / Mashtalir N / Kadoch C / Walz T | |||||||||
| Funding support |  United States, 1 items United States, 1 items
| |||||||||
 Citation Citation |  Journal: Cell / Year: 2020 Journal: Cell / Year: 2020Title: A Structural Model of the Endogenous Human BAF Complex Informs Disease Mechanisms. Authors: Nazar Mashtalir / Hiroshi Suzuki / Daniel P Farrell / Akshay Sankar / Jie Luo / Martin Filipovski / Andrew R D'Avino / Roodolph St Pierre / Alfredo M Valencia / Takashi Onikubo / Robert G ...Authors: Nazar Mashtalir / Hiroshi Suzuki / Daniel P Farrell / Akshay Sankar / Jie Luo / Martin Filipovski / Andrew R D'Avino / Roodolph St Pierre / Alfredo M Valencia / Takashi Onikubo / Robert G Roeder / Yan Han / Yuan He / Jeffrey A Ranish / Frank DiMaio / Thomas Walz / Cigall Kadoch /  Abstract: Mammalian SWI/SNF complexes are ATP-dependent chromatin remodeling complexes that regulate genomic architecture. Here, we present a structural model of the endogenously purified human canonical BAF ...Mammalian SWI/SNF complexes are ATP-dependent chromatin remodeling complexes that regulate genomic architecture. Here, we present a structural model of the endogenously purified human canonical BAF complex bound to the nucleosome, generated using cryoelectron microscopy (cryo-EM), cross-linking mass spectrometry, and homology modeling. BAF complexes bilaterally engage the nucleosome H2A/H2B acidic patch regions through the SMARCB1 C-terminal α-helix and the SMARCA4/2 C-terminal SnAc/post-SnAc regions, with disease-associated mutations in either causing attenuated chromatin remodeling activities. Further, we define changes in BAF complex architecture upon nucleosome engagement and compare the structural model of endogenous BAF to those of related SWI/SNF-family complexes. Finally, we assign and experimentally interrogate cancer-associated hot-spot mutations localizing within the endogenous human BAF complex, identifying those that disrupt BAF subunit-subunit and subunit-nucleosome interfaces in the nucleosome-bound conformation. Taken together, this integrative structural approach provides important biophysical foundations for understanding the mechanisms of BAF complex function in normal and disease states. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_22478.map.gz emd_22478.map.gz | 58.9 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-22478-v30.xml emd-22478-v30.xml emd-22478.xml emd-22478.xml | 11.1 KB 11.1 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_22478_fsc.xml emd_22478_fsc.xml | 9.2 KB | Display |  FSC data file FSC data file |
| Images |  emd_22478.png emd_22478.png | 53.7 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-22478 http://ftp.pdbj.org/pub/emdb/structures/EMD-22478 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-22478 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-22478 | HTTPS FTP |
-Validation report
| Summary document |  emd_22478_validation.pdf.gz emd_22478_validation.pdf.gz | 78.5 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_22478_full_validation.pdf.gz emd_22478_full_validation.pdf.gz | 77.7 KB | Display | |
| Data in XML |  emd_22478_validation.xml.gz emd_22478_validation.xml.gz | 495 B | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-22478 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-22478 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-22478 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-22478 | HTTPS FTP |
-Related structure data
| Related structure data | C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_22478.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_22478.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Cryo-EM map of the human BAF-nucleosome complex refined with a mask for the nucleosome and the ATPase-ARP modules | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.699 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
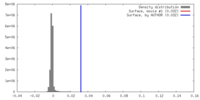
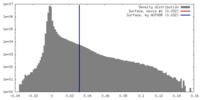
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
- Sample components
Sample components
-Entire : BAF-nucleosome complex
| Entire | Name: BAF-nucleosome complex |
|---|---|
| Components |
|
-Supramolecule #1: BAF-nucleosome complex
| Supramolecule | Name: BAF-nucleosome complex / type: complex / ID: 1 / Parent: 0 |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Recombinant expression | Organism:  |
| Molecular weight | Theoretical: 1.4 MDa |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.5 |
|---|---|
| Grid | Support film - #0 - Film type ID: 1 / Support film - #0 - Material: CARBON / Support film - #0 - topology: HOLEY ARRAY / Support film - #1 - Film type ID: 2 / Support film - #1 - Material: CARBON / Support film - #1 - topology: CONTINUOUS / Details: unspecified |
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 277 K |
- Electron microscopy
Electron microscopy
| Microscope | FEI TALOS ARCTICA |
|---|---|
| Image recording | Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Detector mode: SUPER-RESOLUTION / Number real images: 14841 / Average exposure time: 15.0 sec. / Average electron dose: 69.0 e/Å2 |
| Electron beam | Acceleration voltage: 200 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Talos Arctica / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller














 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)