[English] 日本語
 Yorodumi
Yorodumi- EMDB-22384: Full-length three-dimensional structure of the influenza A virus ... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-22384 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | Full-length three-dimensional structure of the influenza A virus M1 protein and its organization into a matrix layer | ||||||||||||
 Map data Map data | |||||||||||||
 Sample Sample |
| ||||||||||||
 Keywords Keywords | influenza A / virus / matrix layer / M1 protein / VIRAL PROTEIN | ||||||||||||
| Function / homology |  Function and homology information Function and homology informationAssembly of Viral Components at the Budding Site / Influenza Infection / Fusion of the Influenza Virion to the Host Cell Endosome / Release / Budding / Packaging of Eight RNA Segments / Uncoating of the Influenza Virion / Entry of Influenza Virion into Host Cell via Endocytosis / Viral RNP Complexes in the Host Cell Nucleus / NEP/NS2 Interacts with the Cellular Export Machinery ...Assembly of Viral Components at the Budding Site / Influenza Infection / Fusion of the Influenza Virion to the Host Cell Endosome / Release / Budding / Packaging of Eight RNA Segments / Uncoating of the Influenza Virion / Entry of Influenza Virion into Host Cell via Endocytosis / Viral RNP Complexes in the Host Cell Nucleus / NEP/NS2 Interacts with the Cellular Export Machinery / Viral mRNA Translation / viral budding from plasma membrane / structural constituent of virion / host cell nucleus / virion membrane / RNA binding / extracellular region / membrane / plasma membrane Similarity search - Function | ||||||||||||
| Biological species |  Influenza A virus (A/Puerto Rico/8/1934(H1N1)) / Influenza A virus (A/Puerto Rico/8/1934(H1N1)) /  Influenza A virus (strain A/Puerto Rico/8/1934 H1N1) Influenza A virus (strain A/Puerto Rico/8/1934 H1N1) | ||||||||||||
| Method | helical reconstruction / cryo EM / Resolution: 3.4 Å | ||||||||||||
 Authors Authors | Su Z / Pintilie G | ||||||||||||
| Funding support |  United States, 3 items United States, 3 items
| ||||||||||||
 Citation Citation |  Journal: PLoS Biol / Year: 2020 Journal: PLoS Biol / Year: 2020Title: Full-length three-dimensional structure of the influenza A virus M1 protein and its organization into a matrix layer. Authors: Lisa Selzer / Zhaoming Su / Grigore D Pintilie / Wah Chiu / Karla Kirkegaard /   Abstract: Matrix proteins are encoded by many enveloped viruses, including influenza viruses, herpes viruses, and coronaviruses. Underneath the viral envelope of influenza virus, matrix protein 1 (M1) forms an ...Matrix proteins are encoded by many enveloped viruses, including influenza viruses, herpes viruses, and coronaviruses. Underneath the viral envelope of influenza virus, matrix protein 1 (M1) forms an oligomeric layer critical for particle stability and pH-dependent RNA genome release. However, high-resolution structures of full-length monomeric M1 and the matrix layer have not been available, impeding antiviral targeting and understanding of the pH-dependent transitions involved in cell entry. Here, purification and extensive mutagenesis revealed protein-protein interfaces required for the formation of multilayered helical M1 oligomers similar to those observed in virions exposed to the low pH of cell entry. However, single-layered helical oligomers with biochemical and ultrastructural similarity to those found in infectious virions before cell entry were observed upon mutation of a single amino acid. The highly ordered structure of the single-layered oligomers and their likeness to the matrix layer of intact virions prompted structural analysis by cryo-electron microscopy (cryo-EM). The resulting 3.4-Å-resolution structure revealed the molecular details of M1 folding and its organization within the single-shelled matrix. The solution of the full-length M1 structure, the identification of critical assembly interfaces, and the development of M1 assembly assays with purified proteins are crucial advances for antiviral targeting of influenza viruses. #1:  Journal: Plos Biol. / Year: 2020 Journal: Plos Biol. / Year: 2020Title: Full-length three-dimensional structure of the influenza A virus M1 protein and its organization into a matrix layer Authors: Selzer L / Su Z / Pintilie G / Chiu W / Kirkegaard K | ||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_22384.map.gz emd_22384.map.gz | 71 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-22384-v30.xml emd-22384-v30.xml emd-22384.xml emd-22384.xml | 18 KB 18 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_22384.png emd_22384.png | 287.8 KB | ||
| Filedesc metadata |  emd-22384.cif.gz emd-22384.cif.gz | 6.3 KB | ||
| Others |  emd_22384_additional.map.gz emd_22384_additional.map.gz emd_22384_additional_1.map.gz emd_22384_additional_1.map.gz | 658.3 KB 658.3 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-22384 http://ftp.pdbj.org/pub/emdb/structures/EMD-22384 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-22384 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-22384 | HTTPS FTP |
-Validation report
| Summary document |  emd_22384_validation.pdf.gz emd_22384_validation.pdf.gz | 558.8 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_22384_full_validation.pdf.gz emd_22384_full_validation.pdf.gz | 558.4 KB | Display | |
| Data in XML |  emd_22384_validation.xml.gz emd_22384_validation.xml.gz | 7.7 KB | Display | |
| Data in CIF |  emd_22384_validation.cif.gz emd_22384_validation.cif.gz | 8.7 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-22384 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-22384 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-22384 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-22384 | HTTPS FTP |
-Related structure data
| Related structure data |  7jm3MC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_22384.map.gz / Format: CCP4 / Size: 421.9 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_22384.map.gz / Format: CCP4 / Size: 421.9 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.82 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
-Additional map: Segmented M1 protein
| File | emd_22384_additional.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Segmented M1 protein | ||||||||||||
| Projections & Slices |
| ||||||||||||
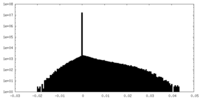
| Density Histograms |
-Additional map: Segmented M1 protein
| File | emd_22384_additional_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Segmented M1 protein | ||||||||||||
| Projections & Slices |
| ||||||||||||
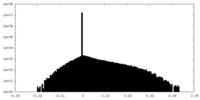
| Density Histograms |
- Sample components
Sample components
-Entire : M1-V97K oligomer
| Entire | Name: M1-V97K oligomer |
|---|---|
| Components |
|
-Supramolecule #1: M1-V97K oligomer
| Supramolecule | Name: M1-V97K oligomer / type: complex / ID: 1 / Parent: 0 / Macromolecule list: all |
|---|---|
| Source (natural) | Organism:  Influenza A virus (A/Puerto Rico/8/1934(H1N1)) Influenza A virus (A/Puerto Rico/8/1934(H1N1)) |
| Molecular weight | Theoretical: 143 kDa/nm |
-Macromolecule #1: Matrix protein 1
| Macromolecule | Name: Matrix protein 1 / type: protein_or_peptide / ID: 1 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Influenza A virus (strain A/Puerto Rico/8/1934 H1N1) Influenza A virus (strain A/Puerto Rico/8/1934 H1N1)Strain: A/Puerto Rico/8/1934 H1N1 |
| Molecular weight | Theoretical: 27.827152 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: SLLTEVETYV LSIIPSGPLK AEIAQRLEDV FAGKNTDLEV LMEWLKTRPI LSPLTKGILG FVFTLTVPSE RGLQRRRFVQ NALNGNGDP NNMDKAKKLY RKLKREITFH GAKEISLSYS AGALASCMGL IYNRMGAVTT EVAFGLVCAT CEQIADSQHR S HRQMVTTT ...String: SLLTEVETYV LSIIPSGPLK AEIAQRLEDV FAGKNTDLEV LMEWLKTRPI LSPLTKGILG FVFTLTVPSE RGLQRRRFVQ NALNGNGDP NNMDKAKKLY RKLKREITFH GAKEISLSYS AGALASCMGL IYNRMGAVTT EVAFGLVCAT CEQIADSQHR S HRQMVTTT NPLIRHENRM VLASTTAKAM EQMAGSSEQA AEAMEVASQA RQMVQAMRTI GTHPSSSAGL KNDLLENLQA YQ KRMGVQM QRFK UniProtKB: Matrix protein 1 |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | helical reconstruction |
| Aggregation state | filament |
- Sample preparation
Sample preparation
| Concentration | 5 mg/mL | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Buffer | pH: 7 Component:
| |||||||||
| Grid | Model: Quantifoil R1.2/1.3 / Material: COPPER / Mesh: 200 / Pretreatment - Type: GLOW DISCHARGE / Pretreatment - Time: 30 sec. / Pretreatment - Atmosphere: AIR / Pretreatment - Pressure: 0.039 kPa | |||||||||
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 277 K / Instrument: FEI VITROBOT MARK IV / Details: Blot for 3 seconds once before plunging.. | |||||||||
| Details | Influenza A virus matrix protein with V97K mutation assembled in vitro |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Temperature | Min: 78.0 K / Max: 78.0 K |
| Specialist optics | Energy filter - Name: GIF Bioquantum / Energy filter - Slit width: 20 eV |
| Image recording | Film or detector model: GATAN K2 QUANTUM (4k x 4k) / Detector mode: COUNTING / Digitization - Dimensions - Width: 3838 pixel / Digitization - Dimensions - Height: 3710 pixel / Digitization - Frames/image: 1-20 / Number grids imaged: 2 / Number real images: 440 / Average exposure time: 4.0 sec. / Average electron dose: 36.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | C2 aperture diameter: 70.0 µm / Calibrated defocus max: 4.3 µm / Calibrated defocus min: 0.3 µm / Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm / Nominal magnification: 165000 |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
- Image processing
Image processing
| Final reconstruction | Number classes used: 200 Applied symmetry - Helical parameters - Δz: 1.96 Å Applied symmetry - Helical parameters - Δ&Phi: 17.1 ° Applied symmetry - Helical parameters - Axial symmetry: C1 (asymmetric) Algorithm: FOURIER SPACE / Resolution.type: BY AUTHOR / Resolution: 3.4 Å / Resolution method: FSC 0.143 CUT-OFF / Software - Name: RELION (ver. 3.0.6) / Number images used: 56602 |
|---|---|
| Segment selection | Number selected: 2268 / Software - Name: EMAN2 (ver. 2.23) |
| Startup model | Type of model: INSILICO MODEL / In silico model: EMAN2.23 Details: Created a cylindrical density map in EMAN2 and used it as an initial model. |
| Final angle assignment | Type: NOT APPLICABLE / Software - Name: RELION (ver. 3.0.6) |
-Atomic model buiding 1
| Initial model | PDB ID: Chain - Source name: PDB / Chain - Initial model type: experimental model |
|---|---|
| Refinement | Space: REAL / Protocol: AB INITIO MODEL / Target criteria: Correlation coefficient |
| Output model |  PDB-7jm3: |
 Movie
Movie Controller
Controller










 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)