[English] 日本語
 Yorodumi
Yorodumi- EMDB-21958: The negative stain EM structure of the human DNA LigI-PCNA-DNA complex -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-21958 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | The negative stain EM structure of the human DNA LigI-PCNA-DNA complex | |||||||||
 Map data Map data | Refined 3D map of full-length human DNA LigI with human PCNA and 32bp non-ligatable nicked DNA. | |||||||||
 Sample Sample |
| |||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | |||||||||
| Method | single particle reconstruction / negative staining / Resolution: 19.0 Å | |||||||||
 Authors Authors | Sverzhinsky A / Pascal JM | |||||||||
| Funding support |  United States, United States,  Canada, 2 items Canada, 2 items
| |||||||||
 Citation Citation |  Journal: J Mol Biol / Year: 2020 Journal: J Mol Biol / Year: 2020Title: Dynamic DNA-bound PCNA complexes co-ordinate Okazaki fragment synthesis, processing and ligation. Authors: Yoshihiro Matsumoto / Rhys C Brooks / Aleksandr Sverzhinsky / John M Pascal / Alan E Tomkinson /   Abstract: More than a million Okazaki fragments are synthesized, processed and joined during replication of the human genome. After synthesis of an RNA-DNA oligonucleotide by DNA polymerase α holoenzyme, ...More than a million Okazaki fragments are synthesized, processed and joined during replication of the human genome. After synthesis of an RNA-DNA oligonucleotide by DNA polymerase α holoenzyme, proliferating cell nuclear antigen (PCNA), a homotrimeric DNA sliding clamp and polymerase processivity factor, is loaded onto the primer-template junction by replication factor C (RFC). Although PCNA interacts with the enzymes DNA polymerase δ (Pol δ), flap endonuclease 1 (FEN1) and DNA ligase I (LigI) that complete Okazaki fragment processing and joining, it is not known how the activities of these enzymes are coordinated. Here we describe a novel interaction between Pol δ and LigI that is critical for Okazaki fragment joining in vitro. Both LigI and FEN1 associate with PCNA-Pol δ during gap-filling synthesis, suggesting that gap-filling synthesis is carried out by a complex of PCNA, Pol δ, FEN1 and LigI. Following ligation, PCNA and LigI remain on the DNA, indicating that Pol δ and FEN1 dissociate during 5' end processing and that LigI engages PCNA at the DNA nick generated by FEN1 and Pol δ. Thus, dynamic PCNA complexes coordinate Okazaki fragment synthesis and processing with PCNA and LigI forming a terminal structure of two linked protein rings encircling the ligated DNA. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_21958.map.gz emd_21958.map.gz | 740 KB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-21958-v30.xml emd-21958-v30.xml emd-21958.xml emd-21958.xml | 15.1 KB 15.1 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_21958.png emd_21958.png | 53.2 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-21958 http://ftp.pdbj.org/pub/emdb/structures/EMD-21958 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-21958 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-21958 | HTTPS FTP |
-Related structure data
| Similar structure data |
|---|
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_21958.map.gz / Format: CCP4 / Size: 1 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_21958.map.gz / Format: CCP4 / Size: 1 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Refined 3D map of full-length human DNA LigI with human PCNA and 32bp non-ligatable nicked DNA. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 3.28 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
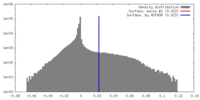
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
- Sample components
Sample components
-Entire : Full-length DNA LigI in complex with nicked duplex DNA and PCNA
| Entire | Name: Full-length DNA LigI in complex with nicked duplex DNA and PCNA |
|---|---|
| Components |
|
-Supramolecule #1: Full-length DNA LigI in complex with nicked duplex DNA and PCNA
| Supramolecule | Name: Full-length DNA LigI in complex with nicked duplex DNA and PCNA type: complex / ID: 1 / Parent: 0 / Macromolecule list: all |
|---|---|
| Molecular weight | Experimental: 220 KDa |
-Supramolecule #2: DNA ligase I
| Supramolecule | Name: DNA ligase I / type: complex / ID: 2 / Parent: 1 / Macromolecule list: #1 |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Recombinant expression | Organism:  |
-Supramolecule #3: Proliferating Cell Nuclear Antigen
| Supramolecule | Name: Proliferating Cell Nuclear Antigen / type: complex / ID: 3 / Parent: 1 / Macromolecule list: #2 |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Recombinant expression | Organism:  |
-Supramolecule #4: Nicked DNA duplex
| Supramolecule | Name: Nicked DNA duplex / type: complex / ID: 4 / Parent: 1 / Macromolecule list: #3 |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Recombinant expression | Organism: synthetic (others) |
-Macromolecule #1: DNA ligase I
| Macromolecule | Name: DNA ligase I / type: protein_or_peptide / ID: 1 / Enantiomer: LEVO / EC number: DNA ligase (ATP) |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Recombinant expression | Organism:  |
| Sequence | String: MGSSHHHHHH SSGLVPRGSH MtRSIMSFFH PKKEGKAKKP EKEASNSSRE TEPPPKAALK EWNGVVSES DSPVKRPGRK AARVLGSEGE EEDEALSPAK GQKPALDCSQ VSPPRPATSP E NNASLSDT SPMDSSPSGI PKRRTARKQL PKRTIQEVLE EQSEDEDREA ...String: MGSSHHHHHH SSGLVPRGSH MtRSIMSFFH PKKEGKAKKP EKEASNSSRE TEPPPKAALK EWNGVVSES DSPVKRPGRK AARVLGSEGE EEDEALSPAK GQKPALDCSQ VSPPRPATSP E NNASLSDT SPMDSSPSGI PKRRTARKQL PKRTIQEVLE EQSEDEDREA KRKKEEEEEE TP KESLTEA EVATEKEGED GDQPTTPPKP LKTSKAETPT ESVSEPEVAT KQELQEEEEQ TKP PRRAPK TLSSFFTPRK PAVKKEVKEE EPGAPGKEGA AEGPLDPSGY NPAKNNYHPV EDAC WKPGQ KVPYLAVART FEKIEEVSAR LRMVETLSNL LRSVVALSPP DLLPVLYLSL NHLGP PQQG LELGVGDGVL LKAVAQATGR QLESVRAEAA EKGDVGLVAE NSRSTQRLML PPPPLT ASG VFSKFRDIAR LTGSASTAKK IDIIKGLFVA CRHSEARFIA RSLSGRLRLG LAEQSVL AA LSQAVSLTPP GQEFPPAMVD AGKGKTAEAR KTWLEEQGMI LKQTFCEVPD LDRIIPVL L EHGLERLPEH CKLSPGIPLK PMLAHPTRGI SEVLKRFEEA AFTCEYKYDG QRAQIHALE GGEVKIFSRN QEDNTGKYPD IISRIPKIKL PSVTSFILDT EAVAWDREKK QIQPFQVLTT RKRKEVDAS EIQVQVCLYA FDLIYLNGES LVREPLSRRR QLLRENFVET EGEFVFATSL D TKDIEQIA EFLEQSVKDS CEGLMVKTLD VDATYEIAKR SHNWLKLKKD YLDGVGDTLD LV VIGAYLG RGKRAGRYGG FLLASYDEDS EELQAICKLG TGFSDEELEE HHQSLKALVL PSP RPYVRI DGAVIPDHWL DPSAVWEVKC ADLSLSPIYP AARGLVDSDK GISLRFPRFI RVRE DKQPE QATTSAQVAC LYRKQSQIQN QQGEDSGSDP EDTY |
-Macromolecule #2: Proliferating Cell Nuclear Antigen
| Macromolecule | Name: Proliferating Cell Nuclear Antigen / type: protein_or_peptide / ID: 2 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Recombinant expression | Organism:  |
| Sequence | String: MGHHHHHHHH HHSSGHIDDD DKHTSLEVVF QGPHMFEARL VQGSILKKVL EALKDLINEA CWDISSSGVN LQSMDSSHVS LVQLTLRSEG FDTYRCDRNL AMGVNLTSMS KILKCAGNED IITLRAEDNA DTLALVFEAP NQEKVSDYEM KLMD LDVEQ LGIPEQEYSC ...String: MGHHHHHHHH HHSSGHIDDD DKHTSLEVVF QGPHMFEARL VQGSILKKVL EALKDLINEA CWDISSSGVN LQSMDSSHVS LVQLTLRSEG FDTYRCDRNL AMGVNLTSMS KILKCAGNED IITLRAEDNA DTLALVFEAP NQEKVSDYEM KLMD LDVEQ LGIPEQEYSC VVKMPSGEFA RICRDLSHIG DAVVISCAKD GVKFSASGEL GNGNI KLSQ TSNVDKEEEA VTIEMNEPVQ LTFALRYLNF FTKATPLSST VTLSMSADVP LVVEYK IAD MGHLKYYLAP KIEDEEGS |
-Macromolecule #3: Nicked DNA duplex
| Macromolecule | Name: Nicked DNA duplex / type: dna / ID: 3 / Classification: DNA |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: GGTTCAGTCC GACGACGCAT CAGCACAGAA GC |
-Experimental details
-Structure determination
| Method | negative staining |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 0.01 mg/mL |
|---|---|
| Buffer | pH: 8 |
| Staining | Type: NEGATIVE / Material: Uranyl Formate |
| Grid | Material: COPPER / Mesh: 300 / Pretreatment - Type: GLOW DISCHARGE / Pretreatment - Atmosphere: AIR |
- Electron microscopy
Electron microscopy
| Microscope | FEI TECNAI 12 |
|---|---|
| Image recording | Film or detector model: FEI EAGLE (4k x 4k) / Average exposure time: 1.0 sec. / Average electron dose: 70.0 e/Å2 |
| Electron beam | Acceleration voltage: 120 kV / Electron source: LAB6 |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal magnification: 67000 |
 Movie
Movie Controller
Controller


 UCSF Chimera
UCSF Chimera






 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)