[English] 日本語
 Yorodumi
Yorodumi- EMDB-21554: Cryo-EM map of the Mycobacterium tuberculosis ClpB disaggregase h... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-21554 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo-EM map of the Mycobacterium tuberculosis ClpB disaggregase hexamer in conformation I in the presence of DnaK chaperone and a model substrate | |||||||||
 Map data Map data | Conformation I | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | ClpB/DnaK / protein aggregates / unfold / Refold protein / CHAPERONE | |||||||||
| Function / homology |  Function and homology information Function and homology informationcellular response to heat / protein refolding / ATP hydrolysis activity / ATP binding / cytoplasm Similarity search - Function | |||||||||
| Biological species |  | |||||||||
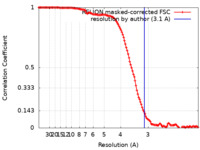
| Method | single particle reconstruction / Resolution: 3.1 Å | |||||||||
 Authors Authors | Yin Y / Li H | |||||||||
| Funding support |  United States, 1 items United States, 1 items
| |||||||||
 Citation Citation |  Journal: Cell Rep / Year: 2021 Journal: Cell Rep / Year: 2021Title: Structural basis for aggregate dissolution and refolding by the Mycobacterium tuberculosis ClpB-DnaK bi-chaperone system. Authors: Yanting Yin / Xiang Feng / Hongjun Yu / Allison Fay / Amanda Kovach / Michael S Glickman / Huilin Li /  Abstract: The M. tuberculosis (Mtb) ClpB is a protein disaggregase that helps to rejuvenate the bacterial cell. DnaK is a protein foldase that can function alone, but it can also bind to the ClpB hexamer to ...The M. tuberculosis (Mtb) ClpB is a protein disaggregase that helps to rejuvenate the bacterial cell. DnaK is a protein foldase that can function alone, but it can also bind to the ClpB hexamer to physically couple protein disaggregation with protein refolding, although the molecular mechanism is not well understood. Here, we report the cryo-EM analysis of the Mtb ClpB-DnaK bi-chaperone in the presence of ATPγS and a protein substrate. We observe three ClpB conformations in the presence of DnaK, identify a conserved TGIP loop linking the oligonucleotide/oligosaccharide-binding domain and the nucleotide-binding domain that is important for ClpB function, derive the interface between the regulatory middle domain of the ClpB and the DnaK nucleotide-binding domain, and find that DnaK binding stabilizes, but does not bend or tilt, the ClpB middle domain. We propose a model for the synergistic actions of aggregate dissolution and refolding by the Mtb ClpB-DnaK bi-chaperone system. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_21554.map.gz emd_21554.map.gz | 164.5 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-21554-v30.xml emd-21554-v30.xml emd-21554.xml emd-21554.xml | 16.7 KB 16.7 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_21554_fsc.xml emd_21554_fsc.xml | 12.7 KB | Display |  FSC data file FSC data file |
| Images |  emd_21554.png emd_21554.png | 99.8 KB | ||
| Filedesc metadata |  emd-21554.cif.gz emd-21554.cif.gz | 6.3 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-21554 http://ftp.pdbj.org/pub/emdb/structures/EMD-21554 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-21554 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-21554 | HTTPS FTP |
-Related structure data
| Related structure data |  6w6gMC  6w6eC  6w6hC  6w6iC  6w6jC  7l6nC C: citing same article ( M: atomic model generated by this map |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_21554.map.gz / Format: CCP4 / Size: 178 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_21554.map.gz / Format: CCP4 / Size: 178 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Conformation I | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.074 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
- Sample components
Sample components
-Entire : ClpB complex with Dnak
| Entire | Name: ClpB complex with Dnak |
|---|---|
| Components |
|
-Supramolecule #1: ClpB complex with Dnak
| Supramolecule | Name: ClpB complex with Dnak / type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1-#2 |
|---|---|
| Source (natural) | Organism:  |
-Macromolecule #1: Chaperone protein ClpB
| Macromolecule | Name: Chaperone protein ClpB / type: protein_or_peptide / ID: 1 / Number of copies: 6 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 92.688281 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MDSFNPTTKT QAALTAALQA ASTAGNPEIR PAHLLMALLT QNDGIAAPLL EAVGVEPATV RAETQRLLDR LPQATGASTQ PQLSRESLA AITTAQQLAT ELDDEYVSTE HVMVGLATGD SDVAKLLTGH GASPQALREA FVKVRGSARV TSPEPEATYQ A LQKYSTDL ...String: MDSFNPTTKT QAALTAALQA ASTAGNPEIR PAHLLMALLT QNDGIAAPLL EAVGVEPATV RAETQRLLDR LPQATGASTQ PQLSRESLA AITTAQQLAT ELDDEYVSTE HVMVGLATGD SDVAKLLTGH GASPQALREA FVKVRGSARV TSPEPEATYQ A LQKYSTDL TARAREGKLD PVIGRDNEIR RVVQVLSRRT KNNPVLIGEP GVGKTAIVEG LAQRIVAGDV PESLRDKTIV AL DLGSMVA GSKYRGEFEE RLKAVLDDIK NSAGQIITFI DELHTIVGAG ATGEGAMDAG NMIKPMLARG ELRLVGATTL DEY RKHIEK DAALERRFQQ VYVGEPSVED TIGILRGLKD RYEVHHGVRI TDSALVAAAT LSDRYITARF LPDKAIDLVD EAAS RLRME IDSRPVEIDE VERLVRRLEI EEMALSKEED EASAERLAKL RSELADQKEK LAELTTRWQN EKNAIEIVRD LKEQL EALR GESERAERDG DLAKAAELRY GRIPEVEKKL DAALPQAQAR EQVMLKEEVG PDDIADVVSA WTGIPAGRLL EGETAK LLR MEDELGKRVI GQKAAVTAVS DAVRRSRAGV SDPNRPTGAF MFLGPTGVGK TELAKALADF LFDDERAMVR IDMSEYG EK HTVARLIGAP PGYVGYEAGG QLTEAVRRRP YTVVLFDEIE KAHPDVFDVL LQVLDEGRLT DGHGRTVDFR NTILILTS N LGSGGSAEQV LAAVRATFKP EFINRLDDVL IFEGLNPEEL VRIVDIQLAQ LGKRLAQRRL QLQVSLPAKR WLAQRGFDP VYGARPLRRL VQQAIGDQLA KMLLAGQVHD GDTVPVNVSP DADSLILG UniProtKB: Chaperone protein ClpB |
-Macromolecule #2: Substrate
| Macromolecule | Name: Substrate / type: protein_or_peptide / ID: 2 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 2.826475 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: (UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK) (UNK)(UNK)(UNK)(UNK)(UNK)(UNK) (UNK)(UNK)(UNK) (UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK) (UNK)(UNK)(UNK) (UNK) |
-Macromolecule #3: PHOSPHOTHIOPHOSPHORIC ACID-ADENYLATE ESTER
| Macromolecule | Name: PHOSPHOTHIOPHOSPHORIC ACID-ADENYLATE ESTER / type: ligand / ID: 3 / Number of copies: 10 / Formula: AGS |
|---|---|
| Molecular weight | Theoretical: 523.247 Da |
| Chemical component information |  ChemComp-AGS: |
-Macromolecule #4: ADENOSINE-5'-DIPHOSPHATE
| Macromolecule | Name: ADENOSINE-5'-DIPHOSPHATE / type: ligand / ID: 4 / Number of copies: 2 / Formula: ADP |
|---|---|
| Molecular weight | Theoretical: 427.201 Da |
| Chemical component information |  ChemComp-ADP: |
-Experimental details
-Structure determination
 Processing Processing | single particle reconstruction |
|---|---|
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 8 |
|---|---|
| Grid | Details: unspecified |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Average electron dose: 2.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: DIFFRACTION |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller



















 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)