+ データを開く
データを開く
- 基本情報
基本情報
| 登録情報 | データベース: EMDB / ID: EMD-2074 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| タイトル | map of the complex of the HA7 Fab with avian flu virus hemagglutinin by negative staining EM | |||||||||
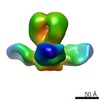
 マップデータ マップデータ | 3D reconstruction from negative staining EM images of the trimeric Fab/hemagglutinin complex | |||||||||
 試料 試料 |
| |||||||||
 キーワード キーワード | HA7 antibody / Fab / hemagglutinin / avian flu virus | |||||||||
| 機能・相同性 | Haemagglutinin, influenzavirus A/B 機能・相同性情報 機能・相同性情報 | |||||||||
| 生物種 |    unidentified influenza virus (インフルエンザウイルス) unidentified influenza virus (インフルエンザウイルス) | |||||||||
| 手法 | 単粒子再構成法 / ネガティブ染色法 / 解像度: 18.0 Å | |||||||||
 データ登録者 データ登録者 | Du L / Jin L / Zhao G / Sun S / Li J / Li Y / Zheng B / Liddington CL / Zhou Y / Jiang S | |||||||||
 引用 引用 |  ジャーナル: J Virol / 年: 2013 ジャーナル: J Virol / 年: 2013タイトル: Identification and structural characterization of a broadly neutralizing antibody targeting a novel conserved epitope on the influenza virus H5N1 hemagglutinin. 著者: Lanying Du / Lei Jin / Guangyu Zhao / Shihui Sun / Junfeng Li / Hong Yu / Ye Li / Bo-Jian Zheng / Robert C Liddington / Yusen Zhou / Shibo Jiang /  要旨: The unabated circulation of the highly pathogenic avian influenza A virus/H5N1 continues to be a serious threat to public health worldwide. Because of the high frequency of naturally occurring ...The unabated circulation of the highly pathogenic avian influenza A virus/H5N1 continues to be a serious threat to public health worldwide. Because of the high frequency of naturally occurring mutations, the emergence of H5N1 variants with high virulence has raised great concerns about the potential transmissibility of the virus in humans. Recent studies have shown that laboratory-mutated or reassortant H5N1 viruses could be efficiently transmitted among mammals, particularly ferrets, the best animal model for humans. Thus, it is critical to establish effective strategies to combat future H5N1 pandemics. In this study, we identified a broadly neutralizing monoclonal antibody (MAb), HA-7, that potently neutralized all tested strains of H5N1 covering clades 0, 1, 2.2, 2.3.4, and 2.3.2.1 and completely protected mice against lethal challenges of H5N1 viruses from clades 1 and 2.3.4. HA-7 specifically targeted the globular head of the H5N1 virus hemagglutinin (HA). Using electron microscopy technology with three-dimensional reconstruction (3D-EM), we discovered that HA-7 bound to a novel and highly conserved conformational epitope that was centered on residues 81 to 83 and 117 to 122 of HA1 (H5 numbering). We further demonstrated that HA-7 inhibited viral entry during postattachment events but not at the receptor-binding step, which is fully consistent with the 3D-EM result. Taken together, we propose that HA-7 could be humanized as an effective passive immunotherapeutic agent for antiviral stockpiling for future influenza pandemics caused by emerging unpredictable H5N1 strains. Our study also provides a sound foundation for the rational design of vaccines capable of inducing broad-spectrum immunity against H5N1. | |||||||||
| 履歴 |
|
- 構造の表示
構造の表示
| ムービー |
 ムービービューア ムービービューア |
|---|---|
| 構造ビューア | EMマップ:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| 添付画像 |
- ダウンロードとリンク
ダウンロードとリンク
-EMDBアーカイブ
| マップデータ |  emd_2074.map.gz emd_2074.map.gz | 117.1 KB |  EMDBマップデータ形式 EMDBマップデータ形式 | |
|---|---|---|---|---|
| ヘッダ (付随情報) |  emd-2074-v30.xml emd-2074-v30.xml emd-2074.xml emd-2074.xml | 10.2 KB 10.2 KB | 表示 表示 |  EMDBヘッダ EMDBヘッダ |
| 画像 |  EMD-2074.png EMD-2074.png | 126.3 KB | ||
| アーカイブディレクトリ |  http://ftp.pdbj.org/pub/emdb/structures/EMD-2074 http://ftp.pdbj.org/pub/emdb/structures/EMD-2074 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-2074 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-2074 | HTTPS FTP |
-検証レポート
| 文書・要旨 |  emd_2074_validation.pdf.gz emd_2074_validation.pdf.gz | 190.9 KB | 表示 |  EMDB検証レポート EMDB検証レポート |
|---|---|---|---|---|
| 文書・詳細版 |  emd_2074_full_validation.pdf.gz emd_2074_full_validation.pdf.gz | 190 KB | 表示 | |
| XML形式データ |  emd_2074_validation.xml.gz emd_2074_validation.xml.gz | 5.2 KB | 表示 | |
| アーカイブディレクトリ |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-2074 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-2074 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-2074 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-2074 | HTTPS FTP |
-関連構造データ
- リンク
リンク
| EMDBのページ |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- マップ
マップ
| ファイル |  ダウンロード / ファイル: emd_2074.map.gz / 形式: CCP4 / 大きさ: 1.4 MB / タイプ: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) ダウンロード / ファイル: emd_2074.map.gz / 形式: CCP4 / 大きさ: 1.4 MB / タイプ: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 注釈 | 3D reconstruction from negative staining EM images of the trimeric Fab/hemagglutinin complex | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 投影像・断面図 | 画像のコントロール
画像は Spider により作成 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ボクセルのサイズ | X=Y=Z: 4.28 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 密度 |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 対称性 | 空間群: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 詳細 | EMDB XML:
CCP4マップ ヘッダ情報:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-添付データ
- 試料の構成要素
試料の構成要素
-全体 : the complex of Fab Fragment of HA7 monoclonal antibody and avian ...
| 全体 | 名称: the complex of Fab Fragment of HA7 monoclonal antibody and avian influenza virus hemagglutinin |
|---|---|
| 要素 |
|
-超分子 #1000: the complex of Fab Fragment of HA7 monoclonal antibody and avian ...
| 超分子 | 名称: the complex of Fab Fragment of HA7 monoclonal antibody and avian influenza virus hemagglutinin タイプ: sample / ID: 1000 / 詳細: The trimeric sample was monodisperse. / 集合状態: one trimeric hemagglutinin binds to three Fab / Number unique components: 2 |
|---|---|
| 分子量 | 実験値: 370 KDa / 理論値: 370 KDa / 手法: based on protein sequences and SDS-PAGE |
-分子 #1: Fab Fragment of HA7 monoclonal antibody
| 分子 | 名称: Fab Fragment of HA7 monoclonal antibody / タイプ: protein_or_peptide / ID: 1 / コピー数: 3 / 集合状態: trimer / 組換発現: Yes |
|---|---|
| 由来(天然) | 生物種:  |
| 組換発現 | 生物種:  |
-分子 #2: Avian influenza virus hemagglutinin
| 分子 | 名称: Avian influenza virus hemagglutinin / タイプ: protein_or_peptide / ID: 2 / コピー数: 3 / 集合状態: trimer / 組換発現: Yes |
|---|---|
| 由来(天然) | 生物種:   unidentified influenza virus (インフルエンザウイルス) unidentified influenza virus (インフルエンザウイルス) |
| 配列 | InterPro: Haemagglutinin, influenzavirus A/B |
-実験情報
-構造解析
| 手法 | ネガティブ染色法 |
|---|---|
 解析 解析 | 単粒子再構成法 |
| 試料の集合状態 | particle |
- 試料調製
試料調製
| 緩衝液 | pH: 7.4 / 詳細: 50mM NaCl, 10mM Tris-HCL,1mM CaCl2, pH7.4 |
|---|---|
| 染色 | タイプ: NEGATIVE 詳細: Grids with adsorbed protein floated on 2% w/v uranyl formate for 30 seconds |
| グリッド | 詳細: 300 mesh copper grid with carbon support, glow discharged in vacuumed air |
| 凍結 | 凍結剤: NONE / 装置: OTHER |
- 電子顕微鏡法
電子顕微鏡法
| 顕微鏡 | FEI TECNAI F20 |
|---|---|
| アライメント法 | Legacy - 非点収差: Objective lens astigmatism was corrected at 70,000 times magnification. |
| 日付 | 2011年8月7日 |
| 撮影 | カテゴリ: CCD フィルム・検出器のモデル: GENERIC TVIPS (4k x 4k) 実像数: 53 / 平均電子線量: 50 e/Å2 / ビット/ピクセル: 8 |
| Tilt angle min | 0 |
| Tilt angle max | 0 |
| 電子線 | 加速電圧: 200 kV / 電子線源:  FIELD EMISSION GUN FIELD EMISSION GUN |
| 電子光学系 | 倍率(補正後): 70000 / 照射モード: FLOOD BEAM / 撮影モード: BRIGHT FIELD / Cs: 2.0 mm / 最大 デフォーカス(公称値): 3.6 µm / 最小 デフォーカス(公称値): 1.2 µm |
| 試料ステージ | 試料ホルダーモデル: SIDE ENTRY, EUCENTRIC |
| 実験機器 |  モデル: Tecnai F20 / 画像提供: FEI Company |
- 画像解析
画像解析
| 詳細 | Image processing was done using EMAN2.The particles were selected by a semi-automatic selection using e2boxer. |
|---|---|
| CTF補正 | 詳細: Each particle |
| 最終 再構成 | 想定した対称性 - 点群: C3 (3回回転対称) / アルゴリズム: OTHER / 解像度のタイプ: BY AUTHOR / 解像度: 18.0 Å / 解像度の算出法: FSC 0.5 CUT-OFF / ソフトウェア - 名称: EMAN2 / 詳細: C3 symmetry was imposed in refinement / 使用した粒子像数: 3172 |
 ムービー
ムービー コントローラー
コントローラー









 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)





















