+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-20306 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | E. coli 50S ribosome bound to compound 40e | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | E. coli ribosome / streptogramin A analog / antibiotics / RIBOSOME | |||||||||
| Function / homology |  Function and homology information Function and homology informationtranscriptional attenuation / endoribonuclease inhibitor activity / RNA-binding transcription regulator activity / negative regulation of cytoplasmic translation / DnaA-L2 complex / translation repressor activity / negative regulation of DNA-templated DNA replication initiation / ribosome assembly / DNA-templated transcription termination / mRNA 5'-UTR binding ...transcriptional attenuation / endoribonuclease inhibitor activity / RNA-binding transcription regulator activity / negative regulation of cytoplasmic translation / DnaA-L2 complex / translation repressor activity / negative regulation of DNA-templated DNA replication initiation / ribosome assembly / DNA-templated transcription termination / mRNA 5'-UTR binding / large ribosomal subunit / transferase activity / ribosomal large subunit assembly / large ribosomal subunit rRNA binding / cytosolic large ribosomal subunit / cytoplasmic translation / negative regulation of translation / rRNA binding / structural constituent of ribosome / ribosome / translation / response to antibiotic / negative regulation of DNA-templated transcription / mRNA binding / DNA binding / RNA binding / zinc ion binding / cytoplasm / cytosol Similarity search - Function | |||||||||
| Biological species |  | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 2.8 Å | |||||||||
 Authors Authors | Pellegrino J / Lee DJ | |||||||||
| Funding support |  United States, 2 items United States, 2 items
| |||||||||
 Citation Citation |  Journal: Nature / Year: 2020 Journal: Nature / Year: 2020Title: Synthetic group A streptogramin antibiotics that overcome Vat resistance. Authors: Qi Li / Jenna Pellegrino / D John Lee / Arthur A Tran / Hector A Chaires / Ruoxi Wang / Jesslyn E Park / Kaijie Ji / David Chow / Na Zhang / Axel F Brilot / Justin T Biel / Gydo van Zundert ...Authors: Qi Li / Jenna Pellegrino / D John Lee / Arthur A Tran / Hector A Chaires / Ruoxi Wang / Jesslyn E Park / Kaijie Ji / David Chow / Na Zhang / Axel F Brilot / Justin T Biel / Gydo van Zundert / Kenneth Borrelli / Dean Shinabarger / Cindy Wolfe / Beverly Murray / Matthew P Jacobson / Estelle Mühle / Olivier Chesneau / James S Fraser / Ian B Seiple /    Abstract: Natural products serve as chemical blueprints for most antibiotics in clinical use. The evolutionary process by which these molecules arise is inherently accompanied by the co-evolution of resistance ...Natural products serve as chemical blueprints for most antibiotics in clinical use. The evolutionary process by which these molecules arise is inherently accompanied by the co-evolution of resistance mechanisms that shorten the clinical lifetime of any given class of antibiotics. Virginiamycin acetyltransferase (Vat) enzymes are resistance proteins that provide protection against streptogramins, potent antibiotics against Gram-positive bacteria that inhibit the bacterial ribosome. Owing to the challenge of selectively modifying the chemically complex, 23-membered macrocyclic scaffold of group A streptogramins, analogues that overcome the resistance conferred by Vat enzymes have not been previously developed. Here we report the design, synthesis, and antibacterial evaluation of group A streptogramin antibiotics with extensive structural variability. Using cryo-electron microscopy and forcefield-based refinement, we characterize the binding of eight analogues to the bacterial ribosome at high resolution, revealing binding interactions that extend into the peptidyl tRNA-binding site and towards synergistic binders that occupy the nascent peptide exit tunnel. One of these analogues has excellent activity against several streptogramin-resistant strains of Staphylococcus aureus, exhibits decreased rates of acetylation in vitro, and is effective at lowering bacterial load in a mouse model of infection. Our results demonstrate that the combination of rational design and modular chemical synthesis can revitalize classes of antibiotics that are limited by naturally arising resistance mechanisms. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_20306.map.gz emd_20306.map.gz | 763.2 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-20306-v30.xml emd-20306-v30.xml emd-20306.xml emd-20306.xml | 19.3 KB 19.3 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_20306.png emd_20306.png | 175 KB | ||
| Filedesc metadata |  emd-20306.cif.gz emd-20306.cif.gz | 7.6 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-20306 http://ftp.pdbj.org/pub/emdb/structures/EMD-20306 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-20306 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-20306 | HTTPS FTP |
-Related structure data
| Related structure data |  6pcsMC  6pc5C  6pc6C  6pc7C  6pc8C  6pchC  6pcqC  6pcrC  6pctC  6wyvC  6x3cC  6x3jC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | |
| EM raw data |  EMPIAR-10523 (Title: E. coli 50S ribosome bound to compound 40e / Data size: 437.6 EMPIAR-10523 (Title: E. coli 50S ribosome bound to compound 40e / Data size: 437.6 Data #1: Unaligned movies of 50S ribosome complex bound to compound 40e [micrographs - multiframe]) |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_20306.map.gz / Format: CCP4 / Size: 824 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_20306.map.gz / Format: CCP4 / Size: 824 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.8283 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
- Sample components
Sample components
-Entire : 50S E. coli ribosome
| Entire | Name: 50S E. coli ribosome |
|---|---|
| Components |
|
-Supramolecule #1: 50S E. coli ribosome
| Supramolecule | Name: 50S E. coli ribosome / type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1-#7 |
|---|---|
| Source (natural) | Organism:  |
-Macromolecule #1: 23S ribosomal RNA
| Macromolecule | Name: 23S ribosomal RNA / type: rna / ID: 1 / Number of copies: 1 |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 941.795562 KDa |
| Sequence | String: GGUUAAGCGA CUAAGCGUAC ACGGUGGAUG CCCUGGCAGU CAGAGGCGAU GAAGGACGUG CUAAUCUGCG AUAAGCGUCG GUAAGGUGA UAUGAACCGU UAUAACCGGC GAUUUCCGAA UGGGGAAACC CAGUGUGUUU CGACACACUA UCAUUAACUG A AUCCAUAG ...String: GGUUAAGCGA CUAAGCGUAC ACGGUGGAUG CCCUGGCAGU CAGAGGCGAU GAAGGACGUG CUAAUCUGCG AUAAGCGUCG GUAAGGUGA UAUGAACCGU UAUAACCGGC GAUUUCCGAA UGGGGAAACC CAGUGUGUUU CGACACACUA UCAUUAACUG A AUCCAUAG GUUAAUGAGG CGAACCGGGG GAACUGAAAC AUCUAAGUAC CCCGAGGAAA AGAAAUCAAC CGAGAUUCCC CC AGUAGCG GCGAGCGAAC GGGGAGCAGC CCAGAGCCUG AAUCAGUGUG UGUGUUAGUG GAAGCGUCUG GAAAGGCGCG CGA UACAGG GUGACAGCCC CGUACACAAA AAUGCACAUG CUGUGAGCUC GAUGAGUAGG GCGGGACACG UGGUAUCCUG UCUG AAUAU GGGGGGACCA UCCUCCAAGG CUAAAUACUC CUGACUGACC GAUAGUGAAC CAGUACCGUG AGGGAAAGGC GAAAA GAAC CCCGGCGAGG GGAGUGAAAA AGAACCUGAA ACCGUGUACG UACAAGCAGU GGGAGCACGC UUAGGCGUGU GACUGC GUA CCUUUUGUAU AAUGGGUCAG CGACUUAUAU UCUGUAGCAA GGUUAACCGA AUAGGGGAGC CGAAGGGAAA CCGAGUC UU AACUGGGCGU UAAGUUGCAG GGUAUAGACC CGAAACCCGG UGAUCUAGCC AUGGGCAGGU UGAAGGUUGG GUAACACU A ACUGGAGGAC CGAACCGACU AAU(1MG)(PSU)(5MU)GAAA AAUUAGCGGA UGACUUGUGG CUGGGGGUGA AAGGCCA AU CAAACCGGGA GAUAGCUGGU UCUCCCCGAA AGCUAUUUAG GUAGCGCCUC GUGAAUUCAU CUCCGGGGGU AGAGCACU G UUUCGGCAAG GGGGUCAUCC CGACUUACCA ACCCGAUGCA AACUGCGAAU ACCGGAGAAU GUUAUCACGG GAGACACAC GGCGGG(PSU)GCU AACGUCCGUC GUGAAGAGGG AAACAACCCA GACCGCCAGC UAAGGUCCCA AAGUCAUGGU UAAGUG GGA AACGAUGUGG GAAGGCCCAG ACAGCCAGGA UGUUGGCUUA GAAGCAGCCA UCAUUUAAAG AAAGCGUAAU AGCUCAC UG GUCGAGUCGG CCUGCGCGGA AGAUGUAACG GGGCUAAACC AUGCACCGAA GCUGCGGCAG CGACGCUUAU GCGUUGUU G GGUAGGGGAG CGUUCUGUAA GCCUGCGAAG GUGUGCUGUG AGGCAUGCUG GAGGUAUCAG AAGUGCGAAU GCUGACAUA AGUAACGAUA AAGCGGGUGA AAAGCCCGCU CGCCGGAAGA CCAAGGGUUC CUGUCCAACG UUAAUCGGGG CAGGGUGAGU CGACCCCUA AGGCGAGGCC GAAAGGCGUA GUCGAUGGGA AACAGGUUAA UAUUCCUGUA CUUGGUGUUA CUGCGAAGGG G GGACGGAG AAGGCUAUGU UGGCCGGGCG ACGGUUGUCC CGGUUUAAGC GUGUAGGCUG GUUUUCCAGG CAAAUCCGGA AA AUCAAGG CUGAGGCGUG AUGACGAGGC ACUACGGUGC UGAAGCAACA AAUGCCCUGC UUCCAGGAAA AGCCUCUAAG CAU CAGGUA ACAUCAAAUC GUACCCCAAA CCGACAC(6MZ)GG UGGUCAGGUA GAGAAUACCA AGGCGCUUGA GAGAACUCGG GUGAAGGAA CUAGGCAAAA UGGUGCCGUA ACUUCGGGAG AAGGCACGCU GAUAUGUAGG UGAGGUCCCU CGCGGAUGGA G CUGAAAUC AGUCGAAGAU ACCAGCUGGC UGCAACUGUU UAUUAAAAAC ACAGCACUGU GCAAACACGA AAGUGGACGU AU ACGGUGU GACGCCU(2MG)CC CGGUGCCGGA AGGUUAAUUG AUGGGGUUAG CGCAAGCGAA GCUCUUGAUC GAAGCCCCG GUAAACGGCG GCCG(PSU)AAC(3TD)A (PSU)AACGGUCCU AAGGUAGCGA AA(5MU)UCCUUGU CGGGUAAGUU CCGA C(5MC)UGC ACGAAUGGCG UAAUGAUGGC CAGGCUGUCU CCACCCGAGA CUCAGUGAAA UUGAACUCGC UGUGAAGAUG C AGUGUACC CGCGGCAAGA CGGAAAGACC CCGU(G7M)AACCU UUACUAUAGC UUGACACUGA ACAUUGAGCC UUGAUGUG U AGGAUAGGUG GGAGGCUUUG AAGUGUGGAC GCCAGUCUGC AUGGAGCCGA CCUUGAAAUA CCACCCUUUA AUGUUUGAU GUUCUAACGU UGACCCGUAA UCCGGGUUGC GGACAGUGUC UGGUGGGUAG UUUGACUG(OMG)G GCGGUCUCCU CCUAAA GAG UAACGGAGGA GCACGAAGGU UGGCUAAUCC UGGUCGGACA UCAGGAGGUU AGUGCAAUGG CAUAAGCCAG CUUGACU GC GAGCGUGACG GCGCGAGCAG GUGCGAAAGC AGGUCAUAGU GAUCCGGUGG UUCUGAAUGG AAGGGCCAUC GCUCAACG G AUAAAAGGUA CUCCG(2MG)GGAU AACAGGC(PSU)GA UACCGCCCAA GAGUUCAUAU CGACGGCGGU GUUUGGCA (OMC) CUCG(2MA)(PSU)GUCG GCUCAUCACA UCCUGGGGCU GAAGUAGGUC CCAAGGGUAU GGC(OMU)GUUCGC CAU UUAAAG UGGUACGCGA GC(PSU)GGGUUUA GAACGUCGUG AGACAGU(PSU)CG GUCCCUAUCU GCCGUGGGCG CUGGAG AAC UGAGGGGGGC UGCUCCUAGU ACGAGAGGAC CGGAGUGGAC GCAUCACUGG UGUUCGGGUU GUCAUGCCAA UGGCACU GC CCGGUAGCUA AAUGCGGAAG AGAUAAGUGC UGAAAGCAUC UAAGCACGAA ACUUGCCCCG AGAUGAGUUC UCCCUGAC C CUUUAAGGGU CCUGAAGGAA CGUUGAAGAC GACGACGUUG AUAGGCCGGG UGUGUAAGCG CAGCGAUGCG UUGAGCUAA CCGGUACUAA UGAACCGUGA GGCUUAACCU U |
-Macromolecule #2: 5S ribosomal RNA
| Macromolecule | Name: 5S ribosomal RNA / type: rna / ID: 2 / Number of copies: 1 |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 38.177762 KDa |
| Sequence | String: GCCUGGCGGC CGUAGCGCGG UGGUCCCACC UGACCCCAUG CCGAACUCAG AAGUGAAACG CCGUAGCGCC GAUGGUAGUG UGGGGUCUC CCCAUGCGAG AGUAGGGAAC UGCCAGGCA GENBANK: GENBANK: CP023870.1 |
-Macromolecule #3: 50S ribosomal protein L2
| Macromolecule | Name: 50S ribosomal protein L2 / type: protein_or_peptide / ID: 3 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 29.663244 KDa |
| Sequence | String: AVVKCKPTSP GRRHVVKVVN PELHKGKPFA PLLEKNSKSG GRNNNGRITT RHIGGGHKQA YRIVDFKRNK DGIPAVVERL EYDPNRSAN IALVLYKDGE RRYILAPKGL KAGDQIQSGV DAAIKPGNTL PMRNIPVGST VHNVEMKPGK GGQLARSAGT Y VQIVARDG ...String: AVVKCKPTSP GRRHVVKVVN PELHKGKPFA PLLEKNSKSG GRNNNGRITT RHIGGGHKQA YRIVDFKRNK DGIPAVVERL EYDPNRSAN IALVLYKDGE RRYILAPKGL KAGDQIQSGV DAAIKPGNTL PMRNIPVGST VHNVEMKPGK GGQLARSAGT Y VQIVARDG AYVTLRLRSG EMRKVEADCR ATLGEVGNAE HMLRVLGKAG AARWRGVRPT VRGTAMNPVD HPHGGGEGRN FG KHPVTPW GVQTKGKKTR SNKRTDKFIV RRRS UniProtKB: Large ribosomal subunit protein uL2 |
-Macromolecule #4: 50S ribosomal protein L15
| Macromolecule | Name: 50S ribosomal protein L15 / type: protein_or_peptide / ID: 4 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 15.008471 KDa |
| Sequence | String: MRLNTLSPAE GSKKAGKRLG RGIGSGLGKT GGRGHKGQKS RSGGGVRRGF EGGQMPLYRR LPKFGFTSRK AAITAEIRLS DLAKVEGGV VDLNTLKAAN IIGIQIEFAK VILAGEVTTP VTVRGLRVTK GARAAIEAAG GKIEE UniProtKB: Large ribosomal subunit protein uL15 |
-Macromolecule #5: 50S ribosomal protein L4
| Macromolecule | Name: 50S ribosomal protein L4 / type: protein_or_peptide / ID: 5 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 22.121566 KDa |
| Sequence | String: MELVLKDAQS ALTVSETTFG RDFNEALVHQ VVVAYAAGAR QGTRAQKTRA EVTGSGKKPW RQKGTGRARS GSIKSPIWRS GGVTFAARP QDHSQKVNKK MYRGALKSIL SELVRQDRLI VVEKFSVEAP KTKLLAQKLK DMALEDVLII TGELDENLFL A ARNLHKVD ...String: MELVLKDAQS ALTVSETTFG RDFNEALVHQ VVVAYAAGAR QGTRAQKTRA EVTGSGKKPW RQKGTGRARS GSIKSPIWRS GGVTFAARP QDHSQKVNKK MYRGALKSIL SELVRQDRLI VVEKFSVEAP KTKLLAQKLK DMALEDVLII TGELDENLFL A ARNLHKVD VRDATGIDPV SLIAFDKVVM TADAVKQVEE MLA UniProtKB: 50S ribosomal protein L4 |
-Macromolecule #6: 50S ribosomal protein L3
| Macromolecule | Name: 50S ribosomal protein L3 / type: protein_or_peptide / ID: 6 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 22.277535 KDa |
| Sequence | String: MIGLVGKKVG MTRIFTEDGV SIPVTVIEVE ANRVTQVKDL ANDGYRAIQV TTGAKKANRV TKPEAGHFAK AGVEAGRGLW EFRLAEGEE FTVGQSISVE LFADVKKVDV TGTSKGKGFA GTVKRWNFRT QDATHGNSLS HRVPGSIGQN QTPGKVFKGK K MAGQMGNE ...String: MIGLVGKKVG MTRIFTEDGV SIPVTVIEVE ANRVTQVKDL ANDGYRAIQV TTGAKKANRV TKPEAGHFAK AGVEAGRGLW EFRLAEGEE FTVGQSISVE LFADVKKVDV TGTSKGKGFA GTVKRWNFRT QDATHGNSLS HRVPGSIGQN QTPGKVFKGK K MAGQMGNE RVTVQSLDVV RVDAERNLLL VKGAVPGATG SDLIVKPAVK A UniProtKB: Large ribosomal subunit protein uL3 |
-Macromolecule #7: 50S ribosomal protein L13
| Macromolecule | Name: 50S ribosomal protein L13 / type: protein_or_peptide / ID: 7 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 16.050606 KDa |
| Sequence | String: MKTFTAKPET VKRDWYVVDA TGKTLGRLAT ELARRLRGKH KAEYTPHVDT GDYIIVLNAD KVAVTGNKRT DKVYYHHTGH IGGIKQATF EEMIARRPER VIEIAVKGML PKGPLGRAMF RKLKVYAGNE HNHAAQQPQV LDI UniProtKB: 50S ribosomal protein L13 |
-Macromolecule #8: (2R)-2-[(3S,4R,5E,10E,12E,14S,26aR)-14-hydroxy-4,12-dimethyl-1,7,...
| Macromolecule | Name: (2R)-2-[(3S,4R,5E,10E,12E,14S,26aR)-14-hydroxy-4,12-dimethyl-1,7,16,22-tetraoxo-4,7,8,9,14,15,16,17,24,25,26,26a-dodecahydro-1H,3H,22H-21,18-(azeno)pyrrolo[2,1-c][1,8,4,19] ...Name: (2R)-2-[(3S,4R,5E,10E,12E,14S,26aR)-14-hydroxy-4,12-dimethyl-1,7,16,22-tetraoxo-4,7,8,9,14,15,16,17,24,25,26,26a-dodecahydro-1H,3H,22H-21,18-(azeno)pyrrolo[2,1-c][1,8,4,19]dioxadiazacyclotetracosin-3-yl]propyl [4-(trifluoromethyl)phenyl]carbamate type: ligand / ID: 8 / Number of copies: 1 / Formula: O8S |
|---|---|
| Molecular weight | Theoretical: 730.727 Da |
| Chemical component information | 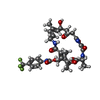 ChemComp-O8S: |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.5 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Average electron dose: 83.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
- Image processing
Image processing
| Startup model | Type of model: NONE |
|---|---|
| Final reconstruction | Resolution.type: BY AUTHOR / Resolution: 2.8 Å / Resolution method: FSC 0.143 CUT-OFF / Number images used: 20644 |
| Initial angle assignment | Type: PROJECTION MATCHING |
| Final angle assignment | Type: PROJECTION MATCHING |
 Movie
Movie Controller
Controller























 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)





















