[English] 日本語
 Yorodumi
Yorodumi- EMDB-20287: The E. coli class-II CAP-dependent transcription activation compl... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-20287 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | The E. coli class-II CAP-dependent transcription activation complex at the state 1 architecture | |||||||||
 Map data Map data | classII CAP-TAC at state 1, image shown at 3.5 RMS | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | class-II / CAP-dependent / transcription activation complex / TRANSCRIPTION / TRANSCRIPTION-DNA complex | |||||||||
| Function / homology |  Function and homology information Function and homology informationsigma factor antagonist complex / RNA polymerase complex / submerged biofilm formation / cellular response to cell envelope stress / regulation of DNA-templated transcription initiation / sigma factor activity / bacterial-type flagellum assembly / bacterial-type RNA polymerase core enzyme binding / cytosolic DNA-directed RNA polymerase complex / bacterial-type flagellum-dependent cell motility ...sigma factor antagonist complex / RNA polymerase complex / submerged biofilm formation / cellular response to cell envelope stress / regulation of DNA-templated transcription initiation / sigma factor activity / bacterial-type flagellum assembly / bacterial-type RNA polymerase core enzyme binding / cytosolic DNA-directed RNA polymerase complex / bacterial-type flagellum-dependent cell motility / nitrate assimilation / cAMP binding / regulation of DNA-templated transcription elongation / transcription elongation factor complex / transcription antitermination / DNA-directed RNA polymerase complex / cell motility / DNA-templated transcription initiation / ribonucleoside binding / DNA-directed RNA polymerase / DNA-directed RNA polymerase activity / response to heat / protein-containing complex assembly / intracellular iron ion homeostasis / protein dimerization activity / transcription cis-regulatory region binding / DNA-binding transcription factor activity / negative regulation of DNA-templated transcription / regulation of DNA-templated transcription / DNA-templated transcription / magnesium ion binding / DNA binding / zinc ion binding / membrane / cytoplasm / cytosol Similarity search - Function | |||||||||
| Biological species |  | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 4.52 Å | |||||||||
 Authors Authors | Liu B / Shi W | |||||||||
 Citation Citation |  Journal: PLoS Biol / Year: 2020 Journal: PLoS Biol / Year: 2020Title: Visualization of two architectures in class-II CAP-dependent transcription activation. Authors: Wei Shi / Yanan Jiang / Yibin Deng / Zigang Dong / Bin Liu /   Abstract: Transcription activation by cyclic AMP (cAMP) receptor protein (CAP) is the classic paradigm of transcription regulation in bacteria. CAP was suggested to activate transcription on class-II promoters ...Transcription activation by cyclic AMP (cAMP) receptor protein (CAP) is the classic paradigm of transcription regulation in bacteria. CAP was suggested to activate transcription on class-II promoters via a recruitment and isomerization mechanism. However, whether and how it modifies RNA polymerase (RNAP) to initiate transcription remains unclear. Here, we report cryo-electron microscopy (cryo-EM) structures of an intact Escherichia coli class-II CAP-dependent transcription activation complex (CAP-TAC) with and without de novo RNA transcript. The structures reveal two distinct architectures of TAC and raise the possibility that CAP binding may induce substantial conformational changes in all the subunits of RNAP and transiently widen the main cleft of RNAP to facilitate DNA promoter entering and formation of the initiation open complex. These structural changes vanish during further RNA transcript synthesis. The observations in this study may reveal a possible on-pathway intermediate and suggest a possibility that CAP activates transcription by inducing intermediate state, in addition to the previously proposed stabilization mechanism. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_20287.map.gz emd_20287.map.gz | 200.4 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-20287-v30.xml emd-20287-v30.xml emd-20287.xml emd-20287.xml | 24 KB 24 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_20287.png emd_20287.png | 227.5 KB | ||
| Filedesc metadata |  emd-20287.cif.gz emd-20287.cif.gz | 8.7 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-20287 http://ftp.pdbj.org/pub/emdb/structures/EMD-20287 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-20287 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-20287 | HTTPS FTP |
-Related structure data
| Related structure data |  6pb5MC  6pb4C  6pb6C M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_20287.map.gz / Format: CCP4 / Size: 216 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_20287.map.gz / Format: CCP4 / Size: 216 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | classII CAP-TAC at state 1, image shown at 3.5 RMS | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.9 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
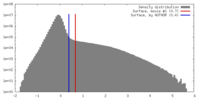
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
- Sample components
Sample components
+Entire : The E coli class-II CAP-dependent transcription activation comple...
+Supramolecule #1: The E coli class-II CAP-dependent transcription activation comple...
+Macromolecule #1: DNA-directed RNA polymerase subunit alpha
+Macromolecule #2: DNA-directed RNA polymerase subunit beta
+Macromolecule #3: DNA-directed RNA polymerase subunit beta'
+Macromolecule #4: DNA-directed RNA polymerase subunit omega
+Macromolecule #5: RNA polymerase sigma factor RpoD
+Macromolecule #6: cAMP-activated global transcriptional regulator CRP
+Macromolecule #7: SYNTHETIC NONTEMPLATE STRAND DNA (78-MER)
+Macromolecule #8: SYNTHETIC TEMPLATE STRAND DNA (78-MER)
+Macromolecule #9: ZINC ION
+Macromolecule #10: MAGNESIUM ION
+Macromolecule #11: ADENOSINE-3',5'-CYCLIC-MONOPHOSPHATE
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 0.55 mg/mL | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Buffer | pH: 7.5 Component:
| ||||||||
| Grid | Model: Quantifoil R1.2/1.3 / Material: COPPER / Mesh: 300 / Support film - Material: CARBON / Support film - topology: HOLEY / Support film - Film thickness: 12 / Pretreatment - Type: GLOW DISCHARGE / Pretreatment - Time: 60 sec. / Pretreatment - Atmosphere: AIR / Details: 15 mA | ||||||||
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 277 K / Instrument: FEI VITROBOT MARK IV / Details: blot for 3 seconds before plunging. |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Temperature | Min: 77.0 K / Max: 77.0 K |
| Image recording | Film or detector model: FEI FALCON III (4k x 4k) / Detector mode: COUNTING / Number grids imaged: 1 / Number real images: 2819 / Average exposure time: 36.0 sec. / Average electron dose: 36.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | C2 aperture diameter: 100.0 µm / Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm / Nominal defocus max: 2.6 µm / Nominal defocus min: 0.8 µm / Nominal magnification: 96000 |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
- Image processing
Image processing
| Startup model | Type of model: NONE |
|---|---|
| Final reconstruction | Resolution.type: BY AUTHOR / Resolution: 4.52 Å / Resolution method: FSC 0.143 CUT-OFF / Software - Name: cisTEM / Number images used: 30296 |
| Initial angle assignment | Type: NOT APPLICABLE |
| Final angle assignment | Type: NOT APPLICABLE |
 Movie
Movie Controller
Controller















 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)