+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo-EM structure of the monocin tail-tube, MttP. | |||||||||
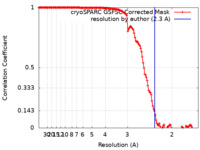
 Map data Map data | Map at 2.3 Angstrom | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | Listeria / monocytogenes / tailocins / TOXIN | |||||||||
| Function / homology | Phage major tail protein TP901-1 / Phage tail tube protein / Antigen A Function and homology information Function and homology information | |||||||||
| Biological species |  Listera (plant) / Listera (plant) /  Listeria monocytogenes 10403S (bacteria) Listeria monocytogenes 10403S (bacteria) | |||||||||
| Method | helical reconstruction / cryo EM / Resolution: 2.3 Å | |||||||||
 Authors Authors | Nadejda S / Lichtenstein R / Schlussel S / Azulay G / Borovok I / Holdengraber V / Elad N / Wolf SG / Zalk R / Zarivach R ...Nadejda S / Lichtenstein R / Schlussel S / Azulay G / Borovok I / Holdengraber V / Elad N / Wolf SG / Zalk R / Zarivach R / Frank GA / Herskovits AA | |||||||||
| Funding support | European Union, 1 items
| |||||||||
 Citation Citation |  Journal: Nat Microbiol / Year: 2024 Journal: Nat Microbiol / Year: 2024Title: Specialized Listeria monocytogenes produce tailocins to provide a population-level competitive growth advantage. Authors: Nadejda Sigal / Rotem Lichtenstein-Wolfheim / Shai Schlussel / Gil Azulay / Ilya Borovok / Vered Holdengraber / Nadav Elad / Sharon G Wolf / Ran Zalk / Raz Zarivach / Gabriel A Frank / Anat A Herskovits /  Abstract: Tailocins are phage tail-like bacteriocins produced by various bacterial species to kill kin competitors. Given that tailocin release is dependent upon cell lysis, regulation of tailocin production ...Tailocins are phage tail-like bacteriocins produced by various bacterial species to kill kin competitors. Given that tailocin release is dependent upon cell lysis, regulation of tailocin production at the single-cell and population level remains unclear. Here we used flow cytometry, competition assays and structural characterization of tailocin production in a human bacterial pathogen, Listeria monocytogenes. We revealed that a specialized subpopulation, constituting less than 1% of the total bacterial population, differentiates to produce, assemble and store thousands of tailocin particles. Tailocins are packed in a highly ordered manner, clustered in a liquid crystalline phase that occupies a substantial volume of the cell. Tailocin production confers a competitive growth advantage for the rest of the population. This study provides molecular insights into tailocin production as a form of altruism, showing how cell specialization within bacterial populations can confer competitive advantages at the population level. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_18416.map.gz emd_18416.map.gz | 230.2 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-18416-v30.xml emd-18416-v30.xml emd-18416.xml emd-18416.xml | 21.4 KB 21.4 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_18416_fsc.xml emd_18416_fsc.xml | 13.1 KB | Display |  FSC data file FSC data file |
| Images |  emd_18416.png emd_18416.png | 114.9 KB | ||
| Filedesc metadata |  emd-18416.cif.gz emd-18416.cif.gz | 6.7 KB | ||
| Others |  emd_18416_half_map_1.map.gz emd_18416_half_map_1.map.gz emd_18416_half_map_2.map.gz emd_18416_half_map_2.map.gz | 226.3 MB 226.3 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-18416 http://ftp.pdbj.org/pub/emdb/structures/EMD-18416 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-18416 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-18416 | HTTPS FTP |
-Related structure data
| Related structure data |  8qhsMC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_18416.map.gz / Format: CCP4 / Size: 244.1 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_18416.map.gz / Format: CCP4 / Size: 244.1 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Map at 2.3 Angstrom | ||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.826 Å | ||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Half map: #1
| File | emd_18416_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: #2
| File | emd_18416_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : Listeria monocytogenes 10403S monocin tail tube comprised of LMRG...
| Entire | Name: Listeria monocytogenes 10403S monocin tail tube comprised of LMRG_02367 tail tube protein (MttP) |
|---|---|
| Components |
|
-Supramolecule #1: Listeria monocytogenes 10403S monocin tail tube comprised of LMRG...
| Supramolecule | Name: Listeria monocytogenes 10403S monocin tail tube comprised of LMRG_02367 tail tube protein (MttP) type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1 |
|---|---|
| Source (natural) | Organism:  Listera (plant) Listera (plant) |
| Molecular weight | Theoretical: 1 kDa/nm |
-Macromolecule #1: Antigen A
| Macromolecule | Name: Antigen A / type: protein_or_peptide / ID: 1 / Number of copies: 5 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Listeria monocytogenes 10403S (bacteria) Listeria monocytogenes 10403S (bacteria) |
| Molecular weight | Theoretical: 18.01026 KDa |
| Recombinant expression | Organism:  Listeria monocytogenes 10403S (bacteria) Listeria monocytogenes 10403S (bacteria) |
| Sequence | String: MAFEENLYCD YTPGAAKAVA GKDVILAVFN AAGDKLLAVA GQQGLTVNRS KDSIEITSKD TVGGWKSKIG GMKEWSIEND GLYVADAES HKELAKYFES DSPVCVKIIN QASKKGLFGG LAIVADYSFE APFDEAMTYS VKLDGMGALV DLTITEGGDQ M PGETPVAP AE UniProtKB: Antigen A |
-Macromolecule #2: water
| Macromolecule | Name: water / type: ligand / ID: 2 / Number of copies: 337 / Formula: HOH |
|---|---|
| Molecular weight | Theoretical: 18.015 Da |
| Chemical component information |  ChemComp-HOH: |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | helical reconstruction |
| Aggregation state | helical array |
- Sample preparation
Sample preparation
| Buffer | pH: 7 |
|---|---|
| Grid | Model: Quantifoil R1.2/1.3 / Material: COPPER / Mesh: 200 / Support film - Material: CARBON / Support film - topology: HOLEY / Pretreatment - Type: GLOW DISCHARGE / Pretreatment - Time: 60 sec. |
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 30 % / Chamber temperature: 20 K / Details: Homemade pneumatic apparatus. |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Specialist optics | Energy filter - Name: GIF Bioquantum / Energy filter - Slit width: 15 eV |
| Image recording | Film or detector model: GATAN K3 BIOQUANTUM (6k x 4k) / Detector mode: COUNTING / Number grids imaged: 1 / Average exposure time: 2.0 sec. / Average electron dose: 49.5 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | C2 aperture diameter: 50.0 µm / Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm / Nominal defocus max: 2.0 µm / Nominal defocus min: 0.8 µm |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller



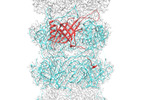
 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)