登録情報 データベース : EMDB / ID : EMD-18324タイトル Respiratory complex I from Paracoccus denitrificans in MSP2N2 nanodiscs Globally sharpened consensus map 複合体 : Respiratory complex Iリガンド : x 13種 / / / 機能・相同性 分子機能 ドメイン・相同性 構成要素
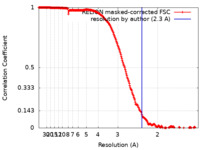
/ / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / 生物種 Paracoccus denitrificans PD1222 (バクテリア)手法 / / 解像度 : 2.3 Å Ivanov BS / Bridges HR / Hirst J 資金援助 Organization Grant number 国 Medical Research Council (MRC, United Kingdom) MC_UU_00015/2 Medical Research Council (MRC, United Kingdom) MC_UU_00028/1
ジャーナル : Nat Commun / 年 : 2024タイトル : Structure of the turnover-ready state of an ancestral respiratory complex I.著者 : Bozhidar S Ivanov / Hannah R Bridges / Owen D Jarman / Judy Hirst / 要旨 : Respiratory complex I is pivotal for cellular energy conversion, harnessing energy from NADH:ubiquinone oxidoreduction to drive protons across energy-transducing membranes for ATP synthesis. Despite ... Respiratory complex I is pivotal for cellular energy conversion, harnessing energy from NADH:ubiquinone oxidoreduction to drive protons across energy-transducing membranes for ATP synthesis. Despite detailed structural information on complex I, its mechanism of catalysis remains elusive due to lack of accompanying functional data for comprehensive structure-function analyses. Here, we present the 2.3-Å resolution structure of complex I from the α-proteobacterium Paracoccus denitrificans, a close relative of the mitochondrial progenitor, in phospholipid-bilayer nanodiscs. Three eukaryotic-type supernumerary subunits (NDUFS4, NDUFS6 and NDUFA12) plus a novel L-isoaspartyl-O-methyltransferase are bound to the core complex. Importantly, the enzyme is in a single, homogeneous resting state that matches the closed, turnover-ready (active) state of mammalian complex I. Our structure reveals the elements that stabilise the closed state and completes P. denitrificans complex I as a unified platform for combining structure, function and genetics in mechanistic studies. 履歴 登録 2023年8月25日 - ヘッダ(付随情報) 公開 2024年9月11日 - マップ公開 2024年9月11日 - 更新 2025年3月26日 - 現状 2025年3月26日 処理サイト : PDBe / 状態 : 公開
すべて表示 表示を減らす
 データを開く
データを開く 基本情報
基本情報
 マップデータ
マップデータ 試料
試料 キーワード
キーワード 機能・相同性情報
機能・相同性情報 Paracoccus denitrificans PD1222 (バクテリア)
Paracoccus denitrificans PD1222 (バクテリア) データ登録者
データ登録者 英国, 2件
英国, 2件  引用
引用 ジャーナル: Nat Commun / 年: 2024
ジャーナル: Nat Commun / 年: 2024


 構造の表示
構造の表示 ダウンロードとリンク
ダウンロードとリンク emd_18324.map.gz
emd_18324.map.gz EMDBマップデータ形式
EMDBマップデータ形式 emd-18324-v30.xml
emd-18324-v30.xml emd-18324.xml
emd-18324.xml EMDBヘッダ
EMDBヘッダ emd_18324_fsc.xml
emd_18324_fsc.xml FSCデータファイル
FSCデータファイル emd_18324.png
emd_18324.png emd_18324_msk_1.map
emd_18324_msk_1.map マスクマップ
マスクマップ emd-18324.cif.gz
emd-18324.cif.gz emd_18324_half_map_1.map.gz
emd_18324_half_map_1.map.gz emd_18324_half_map_2.map.gz
emd_18324_half_map_2.map.gz http://ftp.pdbj.org/pub/emdb/structures/EMD-18324
http://ftp.pdbj.org/pub/emdb/structures/EMD-18324 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-18324
ftp://ftp.pdbj.org/pub/emdb/structures/EMD-18324

 F&H 検索
F&H 検索 リンク
リンク EMDB (EBI/PDBe) /
EMDB (EBI/PDBe) /  EMDataResource
EMDataResource マップ
マップ ダウンロード / ファイル: emd_18324.map.gz / 形式: CCP4 / 大きさ: 1000 MB / タイプ: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES)
ダウンロード / ファイル: emd_18324.map.gz / 形式: CCP4 / 大きさ: 1000 MB / タイプ: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) emd_18324_msk_1.map
emd_18324_msk_1.map 試料の構成要素
試料の構成要素 解析
解析 試料調製
試料調製 電子顕微鏡法
電子顕微鏡法 FIELD EMISSION GUN
FIELD EMISSION GUN
 画像解析
画像解析
 ムービー
ムービー コントローラー
コントローラー


















 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)