[English] 日本語
 Yorodumi
Yorodumi- EMDB-17226: Cryo-EM structure of CBF1-CCAN bound topologically to a centromer... -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo-EM structure of CBF1-CCAN bound topologically to a centromeric CENP-A nucleosome | |||||||||||||||
 Map data Map data | ||||||||||||||||
 Sample Sample |
| |||||||||||||||
 Keywords Keywords | kinetochore / point centromere / CENP-A nucleosome / topological entrapment / centromeric DNA / CELL CYCLE | |||||||||||||||
| Function / homology |  Function and homology information Function and homology informationCbf1-Met4-Met28 complex / positive regulation of sulfate assimilation / regulation of sulfur metabolic process / negative regulation of kinetochore assembly / positive regulation of inositol biosynthetic process / 2-micrometer circle DNA / 2-micrometer plasmid partitioning / negative regulation of meiotic DNA double-strand break formation involved in reciprocal meiotic recombination / COMA complex / maintenance of meiotic sister chromatid cohesion ...Cbf1-Met4-Met28 complex / positive regulation of sulfate assimilation / regulation of sulfur metabolic process / negative regulation of kinetochore assembly / positive regulation of inositol biosynthetic process / 2-micrometer circle DNA / 2-micrometer plasmid partitioning / negative regulation of meiotic DNA double-strand break formation involved in reciprocal meiotic recombination / COMA complex / maintenance of meiotic sister chromatid cohesion / : / HDMs demethylate histones / Mis6-Sim4 complex / : / meiotic sister chromatid segregation / negative regulation of ceramide biosynthetic process / establishment of meiotic sister chromatid cohesion / ascospore formation / HATs acetylate histones / RNA polymerase I upstream activating factor complex / attachment of spindle microtubules to kinetochore / Condensation of Prophase Chromosomes / : / CENP-A containing chromatin assembly / : / centromeric DNA binding / Assembly of the ORC complex at the origin of replication / HDACs deacetylate histones / kinetochore assembly / protein localization to chromosome, centromeric region / outer kinetochore / condensed chromosome, centromeric region / establishment of mitotic sister chromatid cohesion / protein localization to kinetochore / cellular response to methionine / Recruitment and ATM-mediated phosphorylation of repair and signaling proteins at DNA double strand breaks / Oxidative Stress Induced Senescence / spindle pole body / RMTs methylate histone arginines / DNA damage tolerance / SUMOylation of chromatin organization proteins / mitotic spindle assembly checkpoint signaling / RNA Polymerase I Promoter Escape / positive regulation of transcription by RNA polymerase I / nucleolar large rRNA transcription by RNA polymerase I / Estrogen-dependent gene expression / rRNA transcription / mitotic sister chromatid segregation / DNA replication initiation / chromosome, centromeric region / Ub-specific processing proteases / protein localization to CENP-A containing chromatin / CENP-A containing nucleosome / meiotic cell cycle / chromosome segregation / kinetochore / DNA-binding transcription repressor activity, RNA polymerase II-specific / structural constituent of chromatin / peroxisome / heterochromatin formation / nucleosome / mitotic cell cycle / nucleosome assembly / chromatin organization / DNA-binding transcription activator activity, RNA polymerase II-specific / sequence-specific DNA binding / RNA polymerase II-specific DNA-binding transcription factor binding / protein dimerization activity / RNA polymerase II cis-regulatory region sequence-specific DNA binding / chromatin remodeling / DNA-binding transcription factor activity / protein heterodimerization activity / cell division / DNA repair / regulation of DNA-templated transcription / regulation of transcription by RNA polymerase II / protein-containing complex binding / structural molecule activity / negative regulation of transcription by RNA polymerase II / positive regulation of transcription by RNA polymerase II / mitochondrion / DNA binding / identical protein binding / nucleus / cytoplasm Similarity search - Function | |||||||||||||||
| Biological species |  | |||||||||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 3.4 Å | |||||||||||||||
 Authors Authors | Dendooven TD / Zhang Z / Yang J / McLaughlin S / Schwabb J / Scheres S / Yatskevich S / Barford D | |||||||||||||||
| Funding support |  United Kingdom, United Kingdom,  Germany, 4 items Germany, 4 items
| |||||||||||||||
 Citation Citation |  Journal: Sci Adv / Year: 2023 Journal: Sci Adv / Year: 2023Title: Cryo-EM structure of the complete inner kinetochore of the budding yeast point centromere. Authors: Tom Dendooven / Ziguo Zhang / Jing Yang / Stephen H McLaughlin / Johannes Schwab / Sjors H W Scheres / Stanislau Yatskevich / David Barford /  Abstract: The point centromere of budding yeast specifies assembly of the large kinetochore complex to mediate chromatid segregation. Kinetochores comprise the centromere-associated inner kinetochore (CCAN) ...The point centromere of budding yeast specifies assembly of the large kinetochore complex to mediate chromatid segregation. Kinetochores comprise the centromere-associated inner kinetochore (CCAN) complex and the microtubule-binding outer kinetochore KNL1-MIS12-NDC80 (KMN) network. The budding yeast inner kinetochore also contains the DNA binding centromere-binding factor 1 (CBF1) and CBF3 complexes. We determined the cryo-electron microscopy structure of the yeast inner kinetochore assembled onto the centromere-specific centromere protein A nucleosomes (CENP-A). This revealed a central CENP-A with extensively unwrapped DNA ends. These free DNA duplexes bind two CCAN protomers, one of which entraps DNA topologically, positioned on the centromere DNA element I (CDEI) motif by CBF1. The two CCAN protomers are linked through CBF3 forming an arch-like configuration. With a structural mechanism for how CENP-A can also be linked to KMN involving only CENP-QU, we present a model for inner kinetochore assembly onto a point centromere and how it organizes the outer kinetochore for chromosome attachment to the mitotic spindle. | |||||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_17226.map.gz emd_17226.map.gz | 165.2 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-17226-v30.xml emd-17226-v30.xml emd-17226.xml emd-17226.xml | 43.9 KB 43.9 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_17226.png emd_17226.png | 63 KB | ||
| Filedesc metadata |  emd-17226.cif.gz emd-17226.cif.gz | 10.9 KB | ||
| Others |  emd_17226_additional_1.map.gz emd_17226_additional_1.map.gz emd_17226_half_map_1.map.gz emd_17226_half_map_1.map.gz emd_17226_half_map_2.map.gz emd_17226_half_map_2.map.gz | 106.7 MB 165.5 MB 165.4 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-17226 http://ftp.pdbj.org/pub/emdb/structures/EMD-17226 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-17226 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-17226 | HTTPS FTP |
-Related structure data
| Related structure data |  8ow0MC  8ovwC  8ovxC  8ow1C M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_17226.map.gz / Format: CCP4 / Size: 178 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_17226.map.gz / Format: CCP4 / Size: 178 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.853 Å | ||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Additional map: #1
| File | emd_17226_additional_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
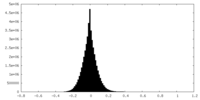
| Density Histograms |
-Half map: #2
| File | emd_17226_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
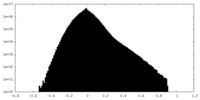
| Density Histograms |
-Half map: #1
| File | emd_17226_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
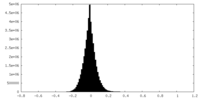
| Density Histograms |
- Sample components
Sample components
+Entire : A complex of CBF1-CCAN bound to centromeric C0N3 DNA
+Supramolecule #1: A complex of CBF1-CCAN bound to centromeric C0N3 DNA
+Macromolecule #1: C0N3 DNA
+Macromolecule #2: C0N3 DNA
+Macromolecule #3: Histone H4
+Macromolecule #4: Histone H2B.1
+Macromolecule #5: Histone H3-like centromeric protein CSE4
+Macromolecule #6: Histone H2A.1
+Macromolecule #7: Centromere-binding protein 1
+Macromolecule #8: Inner kinetochore subunit MCM16
+Macromolecule #9: Inner kinetochore subunit CTF3
+Macromolecule #10: Inner kinetochore subunit MCM22
+Macromolecule #11: Inner kinetochore subunit IML3
+Macromolecule #12: Inner kinetochore subunit CHL4
+Macromolecule #13: Inner kinetochore subunit MCM21
+Macromolecule #14: Inner kinetochore subunit CTF19
+Macromolecule #15: Inner kinetochore subunit OKP1
+Macromolecule #16: Inner kinetochore subunit CNN1
+Macromolecule #17: Inner kinetochore subunit AME1
+Macromolecule #18: Inner kinetochore subunit WIP1
+Macromolecule #19: Inner kinetochore subunit NKP1
+Macromolecule #20: Inner kinetochore subunit NKP2
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 8 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 BIOQUANTUM (6k x 4k) / Average electron dose: 40.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: SPOT SCAN / Imaging mode: BRIGHT FIELD / Nominal defocus max: 2.6 µm / Nominal defocus min: 1.0 µm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller
























 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)










































 Trichoplusia ni (cabbage looper)
Trichoplusia ni (cabbage looper)