[English] 日本語
 Yorodumi
Yorodumi- EMDB-17196: Structure of the protein cage composed of 12 identical 12-membere... -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Structure of the protein cage composed of 12 identical 12-membered protein rings | |||||||||
 Map data Map data | main map | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | protein cage / TRAP protein / VIRUS LIKE PARTICLE | |||||||||
| Function / homology | Transcription attenuation protein MtrB / Tryptophan RNA-binding attenuator protein domain / Tryptophan RNA-binding attenuator protein / Tryptophan RNA-binding attenuator protein-like domain superfamily / DNA-templated transcription termination / regulation of DNA-templated transcription / RNA binding / Transcription attenuation protein MtrB Function and homology information Function and homology information | |||||||||
| Biological species |  Halalkalibacterium halodurans C-125 (bacteria) Halalkalibacterium halodurans C-125 (bacteria) | |||||||||
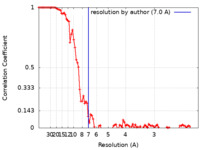
| Method | single particle reconstruction / cryo EM / Resolution: 7.0 Å | |||||||||
 Authors Authors | Biela AP | |||||||||
| Funding support |  Poland, 1 items Poland, 1 items
| |||||||||
 Citation Citation |  Journal: J Mater Chem B / Year: 2024 Journal: J Mater Chem B / Year: 2024Title: An artificial protein cage made from a 12-membered ring. Authors: Izabela Stupka / Artur P Biela / Bernard Piette / Agnieszka Kowalczyk / Karolina Majsterkiewicz / Kinga Borzęcka-Solarz / Antonina Naskalska / Jonathan G Heddle /   Abstract: Artificial protein cages have great potential in diverse fields including as vaccines and drug delivery vehicles. TRAP-cage is an artificial protein cage notable for the way in which the interface ...Artificial protein cages have great potential in diverse fields including as vaccines and drug delivery vehicles. TRAP-cage is an artificial protein cage notable for the way in which the interface between its ring-shaped building blocks can be modified such that the conditions under which cages disassemble can be controlled. To date, TRAP-cages have been constructed from homo-11mer rings, , hendecamers. This is interesting as convex polyhedra with identical regular faces cannot be formed from hendecamers. TRAP-cage overcomes this limitation due to intrinsic flexibility, allowing slight deformation to absorb any error. The resulting TRAP-cage made from 24 TRAP 11mer rings is very close to regular with only very small errors necessary to allow the cage to form. The question arises as to the limits of the error that can be absorbed by a protein structure in this way before the formation of an apparently regular convex polyhedral becomes impossible. Here we use a naturally occurring TRAP variant consisting of twelve identical monomers (, a dodecamer) to probe these limits. We show that it is able to form an apparently regular protein cage consisting of twelve TRAP rings. Comparison of the cryo-EM structure of the new cage with theoretical models and related cages gives insight into the rules of cage formation and allows us to predict other cages that may be formed given TRAP-rings consisting of different numbers of monomers. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_17196.map.gz emd_17196.map.gz | 70.2 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-17196-v30.xml emd-17196-v30.xml emd-17196.xml emd-17196.xml | 15.5 KB 15.5 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_17196_fsc.xml emd_17196_fsc.xml | 11.6 KB | Display |  FSC data file FSC data file |
| Images |  emd_17196.png emd_17196.png | 59.9 KB | ||
| Filedesc metadata |  emd-17196.cif.gz emd-17196.cif.gz | 4.9 KB | ||
| Others |  emd_17196_half_map_1.map.gz emd_17196_half_map_1.map.gz emd_17196_half_map_2.map.gz emd_17196_half_map_2.map.gz | 68.8 MB 68.7 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-17196 http://ftp.pdbj.org/pub/emdb/structures/EMD-17196 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-17196 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-17196 | HTTPS FTP |
-Related structure data
| Related structure data | C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_17196.map.gz / Format: CCP4 / Size: 75.1 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_17196.map.gz / Format: CCP4 / Size: 75.1 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | main map | ||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.065 Å | ||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Half map: hlaf map B
| File | emd_17196_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | hlaf map B | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: half map A
| File | emd_17196_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | half map A | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : Transcription attenuation protein MtrB
| Entire | Name: Transcription attenuation protein MtrB |
|---|---|
| Components |
|
-Supramolecule #1: Transcription attenuation protein MtrB
| Supramolecule | Name: Transcription attenuation protein MtrB / type: complex / ID: 1 / Parent: 0 / Macromolecule list: all Details: protein cage composed of 12 identical 12-membered TRAP rings |
|---|---|
| Source (natural) | Organism:  Halalkalibacterium halodurans C-125 (bacteria) Halalkalibacterium halodurans C-125 (bacteria) |
| Molecular weight | Theoretical: 1.2 MDa |
-Macromolecule #1: Transcription attenuation protein MtrB
| Macromolecule | Name: Transcription attenuation protein MtrB / type: protein_or_peptide / ID: 1 / Details: single point mutation at position 37 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Halalkalibacterium halodurans C-125 (bacteria) Halalkalibacterium halodurans C-125 (bacteria) |
| Recombinant expression | Organism:  |
| Sequence | String: MNVGDNSNFF VIKAKENGVN VFGMTRGTDT RFHHSECLDK GEVMIAQFTE HTSAVKIRGK AIIQTSYGTL DTEKDE UniProtKB: Transcription attenuation protein MtrB |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 1 mg/mL |
|---|---|
| Buffer | pH: 7.4 |
| Grid | Model: Quantifoil R2/2 / Material: COPPER / Mesh: 300 / Support film - Material: CARBON / Support film - topology: HOLEY ARRAY / Pretreatment - Type: GLOW DISCHARGE / Pretreatment - Time: 70 sec. / Pretreatment - Atmosphere: AIR |
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 277 K / Instrument: FEI VITROBOT MARK IV |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: FEI FALCON IV (4k x 4k) / Average electron dose: 40.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: 3.5 µm / Nominal defocus min: 1.5 µm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller




 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)