+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Heterotrimeric Complex of Human ASCT2 with Syncytin-1 | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | Small neutral amino acid transporter / ASCT2 / Syncytin-1 / Receptor binding domain / PROTEIN TRANSPORT | |||||||||
| Function / homology |  Function and homology information Function and homology informationsyncytium formation by plasma membrane fusion / syncytium formation / glutamine secretion / L-glutamine import across plasma membrane / L-glutamine transmembrane transporter activity / glutamine transport / L-serine transmembrane transporter activity / ligand-gated channel activity / neutral amino acid transport / L-aspartate transmembrane transporter activity ...syncytium formation by plasma membrane fusion / syncytium formation / glutamine secretion / L-glutamine import across plasma membrane / L-glutamine transmembrane transporter activity / glutamine transport / L-serine transmembrane transporter activity / ligand-gated channel activity / neutral amino acid transport / L-aspartate transmembrane transporter activity / L-aspartate import across plasma membrane / neutral L-amino acid transmembrane transporter activity / myoblast fusion / Amino acid transport across the plasma membrane / amino acid transmembrane transporter activity / symporter activity / antiporter activity / RHOJ GTPase cycle / protein homotrimerization / RHOQ GTPase cycle / amino acid transport / RHOH GTPase cycle / RAC3 GTPase cycle / anatomical structure morphogenesis / transport across blood-brain barrier / RAC1 GTPase cycle / basal plasma membrane / erythrocyte differentiation / centriolar satellite / melanosome / signaling receptor activity / virus receptor activity / ciliary basal body / extracellular exosome / metal ion binding / membrane / plasma membrane Similarity search - Function | |||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | |||||||||
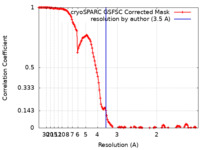
| Method | single particle reconstruction / cryo EM / Resolution: 3.5 Å | |||||||||
 Authors Authors | Khare S / Reyes N | |||||||||
| Funding support | European Union, 1 items
| |||||||||
 Citation Citation |  Journal: Nat Struct Mol Biol / Year: 2024 Journal: Nat Struct Mol Biol / Year: 2024Title: Receptor-recognition and antiviral mechanisms of retrovirus-derived human proteins. Authors: Shashank Khare / Miryam I Villalba / Juan C Canul-Tec / Arantza Balsebre Cajiao / Anand Kumar / Marija Backovic / Felix A Rey / Els Pardon / Jan Steyaert / Camilo Perez / Nicolas Reyes /     Abstract: Human syncytin-1 and suppressyn are cellular proteins of retroviral origin involved in cell-cell fusion events to establish the maternal-fetal interface in the placenta. In cell culture, they ...Human syncytin-1 and suppressyn are cellular proteins of retroviral origin involved in cell-cell fusion events to establish the maternal-fetal interface in the placenta. In cell culture, they restrict infections from members of the largest interference group of vertebrate retroviruses, and are regarded as host immunity factors expressed during development. At the core of the syncytin-1 and suppressyn functions are poorly understood mechanisms to recognize a common cellular receptor, the membrane transporter ASCT2. Here, we present cryo-electron microscopy structures of human ASCT2 in complexes with the receptor-binding domains of syncytin-1 and suppressyn. Despite their evolutionary divergence, the two placental proteins occupy similar positions in ASCT2, and are stabilized by the formation of a hybrid β-sheet or 'clamp' with the receptor. Structural predictions of the receptor-binding domains of extant retroviruses indicate overlapping binding interfaces and clamping sites with ASCT2, revealing a competition mechanism between the placental proteins and the retroviruses. Our work uncovers a common ASCT2 recognition mechanism by a large group of endogenous and disease-causing retroviruses, and provides high-resolution views on how placental human proteins exert morphological and immunological functions. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_17194.map.gz emd_17194.map.gz | 217 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-17194-v30.xml emd-17194-v30.xml emd-17194.xml emd-17194.xml | 21.8 KB 21.8 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_17194_fsc.xml emd_17194_fsc.xml | 13.3 KB | Display |  FSC data file FSC data file |
| Images |  emd_17194.png emd_17194.png | 86.2 KB | ||
| Filedesc metadata |  emd-17194.cif.gz emd-17194.cif.gz | 7 KB | ||
| Others |  emd_17194_half_map_1.map.gz emd_17194_half_map_1.map.gz emd_17194_half_map_2.map.gz emd_17194_half_map_2.map.gz | 226.3 MB 226.3 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-17194 http://ftp.pdbj.org/pub/emdb/structures/EMD-17194 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-17194 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-17194 | HTTPS FTP |
-Related structure data
| Related structure data |  8oujMC  8oudC  8ouhC  8ouiC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_17194.map.gz / Format: CCP4 / Size: 244.1 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_17194.map.gz / Format: CCP4 / Size: 244.1 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.731 Å | ||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Half map: #2
| File | emd_17194_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: #1
| File | emd_17194_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : Complex ASCT2 with Syncytin-1
| Entire | Name: Complex ASCT2 with Syncytin-1 |
|---|---|
| Components |
|
-Supramolecule #1: Complex ASCT2 with Syncytin-1
| Supramolecule | Name: Complex ASCT2 with Syncytin-1 / type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1-#2 |
|---|---|
| Molecular weight | Theoretical: 180 kDa/nm |
-Supramolecule #2: Alanine Serine Cysteine Transporter 2
| Supramolecule | Name: Alanine Serine Cysteine Transporter 2 / type: complex / ID: 2 / Parent: 1 / Macromolecule list: #1 |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
-Supramolecule #3: Syncytin-1
| Supramolecule | Name: Syncytin-1 / type: complex / ID: 3 / Parent: 1 / Macromolecule list: #2 |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
-Macromolecule #1: Neutral amino acid transporter B(0)
| Macromolecule | Name: Neutral amino acid transporter B(0) / type: protein_or_peptide / ID: 1 / Number of copies: 3 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 58.984566 KDa |
| Recombinant expression | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: MWSHPQFEKS SGGLEVLFQG PMVADPPRDS KGLAAAEPTA NGGLALASIE DQGAAAGGYC GSRDQVRRCL RANLLVLLTV VAVVAGVAL GLGVSGAGGA LALGPERLSA FVFPGELLLR LLRMIILPLV VCSLIGGAAS LDPGALGRLG AWALLFFLVT T LLASALGV ...String: MWSHPQFEKS SGGLEVLFQG PMVADPPRDS KGLAAAEPTA NGGLALASIE DQGAAAGGYC GSRDQVRRCL RANLLVLLTV VAVVAGVAL GLGVSGAGGA LALGPERLSA FVFPGELLLR LLRMIILPLV VCSLIGGAAS LDPGALGRLG AWALLFFLVT T LLASALGV GLALALQPGA ASAAINASVG AAGSAENAPS KEVLDSFLDL ARNIFPSNLV SAAFRSYSTT YEERNITGTR VK VPVGQEV EGMNILGLVV FAIVFGVALR KLGPEGELLI RFFNSFNEAT MVLVSWIMWY APVGIMFLVA GKIVEMEDVG LLF ARLGKY ILCCLLGHAI HGLLVLPLIY FLFTRKNPYR FLWGIVTPLA TAFGTSSSSA TLPLMMKCVE ENNGVAKHIS RFIL PIGAT VNMDGAALFQ CVAAVFIAQL SQQSLDFVKI ITILVTATAS SVGAAGIPAG GVLTLAIILE AVNLPVDHIS LILAV DWLV DRSCTVLNVE GDALGAGLLQ NYVDRTESRS TEPELIQVKS ELPLDPLPVP TEEGNPLLKH YRGPAGDATV ASEKES VM UniProtKB: Neutral amino acid transporter B(0) |
-Macromolecule #2: Syncytin-1
| Macromolecule | Name: Syncytin-1 / type: protein_or_peptide / ID: 2 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 49.24593 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: AVVAFVGLSL GAPPPCRCMT SSSPYQEFLW RMQRPGNIDA PSYRSLSKGT PTFTAHTHMP RNCYHSATLC MHANTHYWTG KMINPSCPG GLGVTVCWTY FTQTGMSDGG GVQDQAREKH VKEVISQLTR VHGTSSPYKG LDLSKLHETL RTHTRLVSLF N TTLTGLHE ...String: AVVAFVGLSL GAPPPCRCMT SSSPYQEFLW RMQRPGNIDA PSYRSLSKGT PTFTAHTHMP RNCYHSATLC MHANTHYWTG KMINPSCPG GLGVTVCWTY FTQTGMSDGG GVQDQAREKH VKEVISQLTR VHGTSSPYKG LDLSKLHETL RTHTRLVSLF N TTLTGLHE VSAQNPTNSW ICLPLNFRPY VSIPVPEQWN NFSTEINTTS VLVGPLVSNL EITHTSNLTC VKFSNTTYTT NS QCIRWVT PPTQIVCLPS GIFFVCGTSA YRCLNGSSES MCFLSFLVPP MTIYTEQDLY NYVISKPRNK RVPILPFVIG AGV LGALGT GIGGITTSTQ FYYKLSQELN GDMERVADSL VTLQDQLNSL AAVVLQNRRA LDLLTAERGG TCLFLGEECC YYVN QSGIV TEKVKEIRDR IQRRAEELRN TGPWGSGLEV LFQGPGPEPE A UniProtKB: Syncytin-1 |
-Macromolecule #3: ALANINE
| Macromolecule | Name: ALANINE / type: ligand / ID: 3 / Number of copies: 2 / Formula: ALA |
|---|---|
| Molecular weight | Theoretical: 89.093 Da |
| Chemical component information | 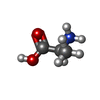 ChemComp-ALA: |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 9 mg/mL | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Buffer | pH: 7.4 Component:
| ||||||||||||||||||
| Grid | Model: Quantifoil R1.2/1.3 / Material: GOLD / Mesh: 300 / Support film - Material: CARBON / Support film - topology: CONTINUOUS / Support film - Film thickness: 10 / Pretreatment - Type: GLOW DISCHARGE / Pretreatment - Time: 40 sec. / Pretreatment - Atmosphere: AIR / Pretreatment - Pressure: 0.003 kPa | ||||||||||||||||||
| Vitrification | Cryogen name: ETHANE-PROPANE / Chamber humidity: 100 % / Chamber temperature: 277 K / Instrument: FEI VITROBOT MARK IV |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Specialist optics | Phase plate: VOLTA PHASE PLATE / Energy filter - Name: TFS Selectris X / Energy filter - Slit width: 10 eV |
| Software | Name: EPU (ver. 2) |
| Image recording | Film or detector model: FEI FALCON IV (4k x 4k) / Number grids imaged: 1 / Number real images: 15376 / Average exposure time: 6.0 sec. / Average electron dose: 53.4 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | C2 aperture diameter: 50.0 µm / Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm / Nominal defocus max: 1.5 µm / Nominal defocus min: 0.4 µm / Nominal magnification: 165000 |
| Sample stage | Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
+ Image processing
Image processing
-Atomic model buiding 1
| Initial model |
| |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Software | Name:  Coot (ver. 0.9.8.3) Coot (ver. 0.9.8.3) | |||||||||
| Refinement | Space: REAL / Protocol: AB INITIO MODEL / Overall B value: 82.2 | |||||||||
| Output model |  PDB-8ouj: |
 Movie
Movie Controller
Controller













 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)