[English] 日本語
 Yorodumi
Yorodumi- EMDB-17131: Knockout of GMC-oxidoreductase genes reveals that functional redu... -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Knockout of GMC-oxidoreductase genes reveals that functional redundancy preserves mimivirus essential functions | |||||||||
 Map data Map data | CL1 masked post-processed main map | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | GMC-oxidoreductase / genomic fiber / mimivirus / STRUCTURAL PROTEIN | |||||||||
| Function / homology |  Function and homology information Function and homology informationoxidoreductase activity, acting on CH-OH group of donors / flavin adenine dinucleotide binding Similarity search - Function | |||||||||
| Biological species |  Mimivirus reunion Mimivirus reunion | |||||||||
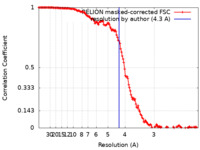
| Method | helical reconstruction / cryo EM / Resolution: 4.3 Å | |||||||||
 Authors Authors | Alempic JM / Bisio H / Villalta A / Santini S / Lartigue A / Schmitt A / Bugnot C / Notaro A / Belmudes L / Adrait A ...Alempic JM / Bisio H / Villalta A / Santini S / Lartigue A / Schmitt A / Bugnot C / Notaro A / Belmudes L / Adrait A / Poirot O / Ptchelkine D / De Castro C / Coute Y / Abergel C | |||||||||
| Funding support | European Union, 1 items
| |||||||||
 Citation Citation |  Journal: Microlife / Year: 2024 Journal: Microlife / Year: 2024Title: Functional redundancy revealed by the deletion of the mimivirus GMC-oxidoreductase genes. Authors: Jean-Marie Alempic / Hugo Bisio / Alejandro Villalta / Sébastien Santini / Audrey Lartigue / Alain Schmitt / Claire Bugnot / Anna Notaro / Lucid Belmudes / Annie Adrait / Olivier Poirot / ...Authors: Jean-Marie Alempic / Hugo Bisio / Alejandro Villalta / Sébastien Santini / Audrey Lartigue / Alain Schmitt / Claire Bugnot / Anna Notaro / Lucid Belmudes / Annie Adrait / Olivier Poirot / Denis Ptchelkine / Cristina De Castro / Yohann Couté / Chantal Abergel /   Abstract: The mimivirus 1.2 Mb genome was shown to be organized into a nucleocapsid-like genomic fiber encased in the nucleoid compartment inside the icosahedral capsid. The genomic fiber protein shell is ...The mimivirus 1.2 Mb genome was shown to be organized into a nucleocapsid-like genomic fiber encased in the nucleoid compartment inside the icosahedral capsid. The genomic fiber protein shell is composed of a mixture of two GMC-oxidoreductase paralogs, one of them being the main component of the glycosylated layer of fibrils at the surface of the virion. In this study, we determined the effect of the deletion of each of the corresponding genes on the genomic fiber and the layer of surface fibrils. First, we deleted the GMC-oxidoreductase, the most abundant in the genomic fiber, and determined its structure and composition in the mutant. As expected, it was composed of the second GMC-oxidoreductase and contained 5- and 6-start helices similar to the wild-type fiber. This result led us to propose a model explaining their coexistence. Then we deleted the GMC-oxidoreductase, the most abundant in the layer of fibrils, to analyze its protein composition in the mutant. Second, we showed that the fitness of single mutants and the double mutant were not decreased compared with the wild-type viruses under laboratory conditions. Third, we determined that deleting the GMC-oxidoreductase genes did not impact the glycosylation or the glycan composition of the layer of surface fibrils, despite modifying their protein composition. Because the glycosylation machinery and glycan composition of members of different clades are different, we expanded the analysis of the protein composition of the layer of fibrils to members of the B and C clades and showed that it was different among the three clades and even among isolates within the same clade. Taken together, the results obtained on two distinct central processes (genome packaging and virion coating) illustrate an unexpected functional redundancy in members of the family , suggesting this may be the major evolutionary force behind their giant genomes. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_17131.map.gz emd_17131.map.gz | 58.2 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-17131-v30.xml emd-17131-v30.xml emd-17131.xml emd-17131.xml | 18.9 KB 18.9 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_17131_fsc.xml emd_17131_fsc.xml | 14.2 KB | Display |  FSC data file FSC data file |
| Images |  emd_17131.png emd_17131.png | 137.4 KB | ||
| Filedesc metadata |  emd-17131.cif.gz emd-17131.cif.gz | 6.6 KB | ||
| Others |  emd_17131_half_map_1.map.gz emd_17131_half_map_1.map.gz emd_17131_half_map_2.map.gz emd_17131_half_map_2.map.gz | 193 MB 192.9 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-17131 http://ftp.pdbj.org/pub/emdb/structures/EMD-17131 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-17131 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-17131 | HTTPS FTP |
-Related structure data
| Related structure data |  8orsMC  8orhC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_17131.map.gz / Format: CCP4 / Size: 244.1 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_17131.map.gz / Format: CCP4 / Size: 244.1 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | CL1 masked post-processed main map | ||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.0859 Å | ||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Half map: CL1 half map 1
| File | emd_17131_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | CL1 half map 1 | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: CL1 half map 2
| File | emd_17131_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | CL1 half map 2 | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : Mimivirus reunion
| Entire | Name:  Mimivirus reunion Mimivirus reunion |
|---|---|
| Components |
|
-Supramolecule #1: Mimivirus reunion
| Supramolecule | Name: Mimivirus reunion / type: virus / ID: 1 / Parent: 0 / Macromolecule list: #1 Details: in the source organism, qu_946 gene has been removed and replaced by a selection cassette. NCBI-ID: 2813486 / Sci species name: Mimivirus reunion / Virus type: VIRUS-LIKE PARTICLE / Virus isolate: STRAIN / Virus enveloped: No / Virus empty: No |
|---|---|
| Host (natural) | Organism:  Acanthamoeba castellanii str. Neff (eukaryote) / Strain: KO_qu946 Acanthamoeba castellanii str. Neff (eukaryote) / Strain: KO_qu946 |
| Virus shell | Shell ID: 1 / Name: genomic fiber / Diameter: 300.0 Å |
-Macromolecule #1: Putative GMC-type oxidoreductase
| Macromolecule | Name: Putative GMC-type oxidoreductase / type: protein_or_peptide / ID: 1 Details: source organism: mimivirus reunion mutant where the qu_946 gene has been replaced by a selection cassette. Number of copies: 2 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Mimivirus reunion / Strain: HB1931 Mimivirus reunion / Strain: HB1931 |
| Molecular weight | Theoretical: 77.018023 KDa |
| Sequence | String: MKNRECCKCY NPCEKICVNY STTDVAFERP NPCKPTPCKP TPIPCDPCHN TKDNLTGDIV IIGAGAAGSL LAHYLARFSN MKIILLEAG HSHFNDPVVT DPMGFFGKYN PPNENISMSQ NPSYSWQGAQ EPNTGAYGNR PIIAHGMGFG GSTMINRLNL V VGGRTVFD ...String: MKNRECCKCY NPCEKICVNY STTDVAFERP NPCKPTPCKP TPIPCDPCHN TKDNLTGDIV IIGAGAAGSL LAHYLARFSN MKIILLEAG HSHFNDPVVT DPMGFFGKYN PPNENISMSQ NPSYSWQGAQ EPNTGAYGNR PIIAHGMGFG GSTMINRLNL V VGGRTVFD NDWPVGWKYD DVKNYFRRVL VDINPVRDNT KASITSVALD ALRIIAEQQI ASGEPVDFLL NKATGNVPNV EK TTPDAVP LNLNDYEGVN SVVAFSSFYM GVNQLSDGNY IRKYAGNTYL NRNYVDENGR GIGKFSGLRV VSDAVVDRII FKG NRAVGV NYIDREGIMH YVKVNKEVVV TSGAFYTPTI LQRSGIGDFT YLSSIGVKNL VYNNPLVGTG LKNHYSPVTI TRVH GEPSE VSRFLSNMAA NPTNMGFKGL AELGFHRLDP NKPANANTVT YRKYQLMMTA GVGIPAEQQY LSGLSPSSNN LFTLI ADDI RFAPEGYIKI GTPNIPRDVP KIFFNTFVTY TPTSAPADQQ WPIAQKTLAP LISALLGYDI IYQTLISMNQ TARDSG FQV SLEMVYPLND LIYKLHNGLA TYGANWWHYF VPTLVGDDTP AGREFADTLS KLSYYPRVGA HLDSHQGCSC SIGRTVD SN LKVIGTQNVR VADLSAAAFP PGGNTWATAS MIGARAVDLI LGFPYLRDLP VNDVPILNVN UniProtKB: GMC-type oxidoreductase |
-Macromolecule #2: FLAVIN-ADENINE DINUCLEOTIDE
| Macromolecule | Name: FLAVIN-ADENINE DINUCLEOTIDE / type: ligand / ID: 2 / Number of copies: 2 / Formula: FAD |
|---|---|
| Molecular weight | Theoretical: 785.55 Da |
| Chemical component information |  ChemComp-FAD: |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | helical reconstruction |
| Aggregation state | filament |
- Sample preparation
Sample preparation
| Buffer | pH: 7.5 / Component - Concentration: 40.0 mmol/l / Component - Formula: Tris-HCl / Component - Name: Tris-HCl |
|---|---|
| Vitrification | Cryogen name: ETHANE-PROPANE / Chamber humidity: 100 % / Chamber temperature: 277 K / Instrument: FEI VITROBOT MARK IV |
| Details | genomic fiber purified on ClCs gradient |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Specialist optics | Energy filter - Name: GIF Bioquantum |
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Digitization - Dimensions - Width: 5760 pixel / Digitization - Dimensions - Height: 4092 pixel / Number real images: 2224 / Average exposure time: 2.3 sec. / Average electron dose: 35.597 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | C2 aperture diameter: 50.0 µm / Illumination mode: OTHER / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm / Nominal defocus max: 2.8000000000000003 µm / Nominal defocus min: 0.6 µm / Nominal magnification: 81000 |
| Sample stage | Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
+ Image processing
Image processing
-Atomic model buiding 1
| Initial model | PDB ID: Chain - Residue range: 4-652 / Chain - Source name: PDB / Chain - Initial model type: experimental model |
|---|---|
| Refinement | Space: REAL / Protocol: RIGID BODY FIT / Overall B value: 86.35 |
| Output model |  PDB-8ors: |
 Movie
Movie Controller
Controller





 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)